J Vet Sci.
2012 Mar;13(1):7-13. 10.4142/jvs.2012.13.1.7.
Elm tree bark extract inhibits HepG2 hepatic cancer cell growth via pro-apoptotic activity
- Affiliations
-
- 1College of Veterinary Medicine, Chungbuk National University, Cheongju 361-763, Korea. bwahn@cbu.ac.kr
- KMID: 1364997
- DOI: http://doi.org/10.4142/jvs.2012.13.1.7
Abstract
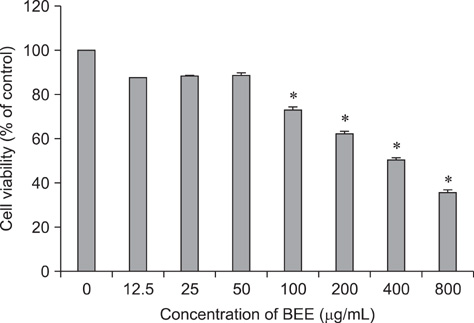
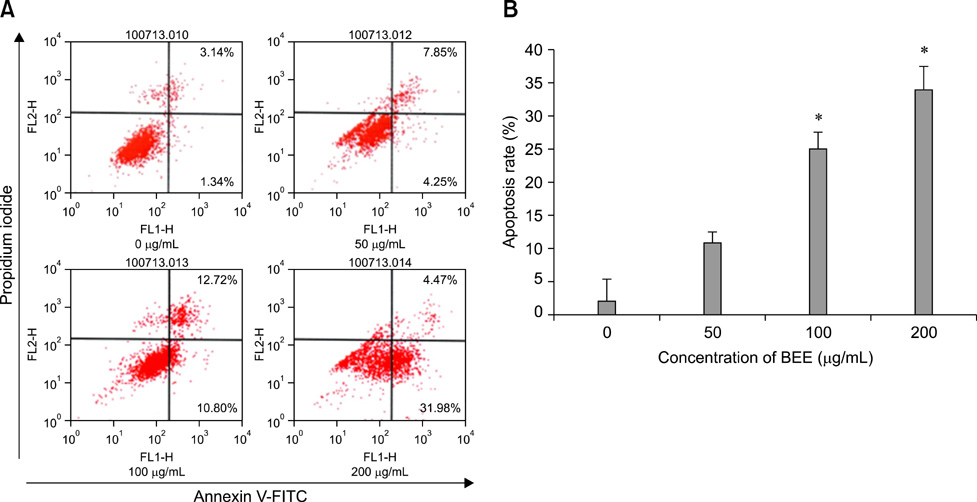
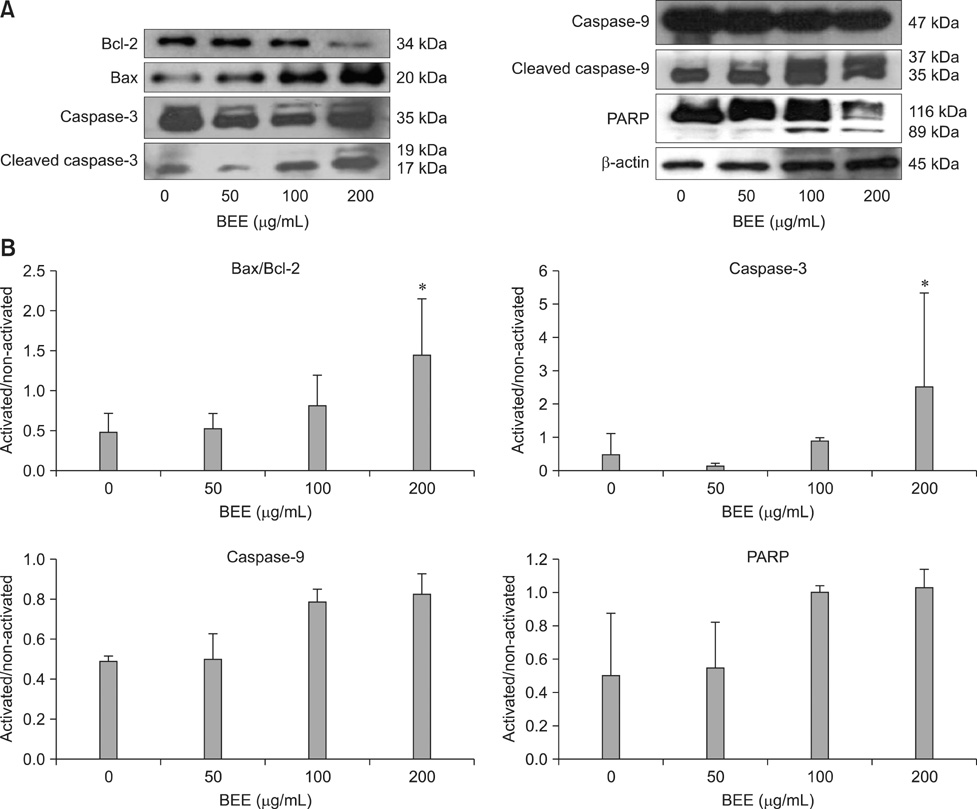
- Control of inflammation is widely accepted as an important strategy for cancer chemoprevention. Anti-inflammatory effects of bark extracts of elm tree (BEE) have been amply reported. Therefore, BEE may be a good candidate cancer chemopreventive agent. Considering the high incidence of hepatic cancer and limited therapeutic approaches for treating this disease, it is important to develop liver cancer-specific chemopreventive agents. To evaluate the chemopreventive potential of BEE, we investigated the growth inhibition effect of BEE on the HepG2 human hepatocellular carcinoma cell line. We performed a cell counting kit-8 assay to determine cell viability, and 4,6-diamino-2-phenylindole staining and flow cytometry to measure apoptotic cell death. Finally, the expression levels of pro- and anti-apoptotic proteins were measured. BEE inhibited the growth of HepG2 cells and induced apoptosis in a dose-dependent manner. Pro-apoptotic activity was promoted via the mitochondrial pathway of apoptosis, as demonstrated by the activation of pro-apoptotic proteins Bax, caspase-9, caspase-3, and poly (ADP-ribose) polymerase as well as the down-regulation of the anti-apoptotic protein Bcl-2. These results suggest that BEE may have potential use in hepatic cancer chemoprevention by suppressing cancer cell growth via pro-apoptotic activity.
Keyword
MeSH Terms
-
Apoptosis/*drug effects
Blotting, Western
Carcinoma, Hepatocellular/*drug therapy/metabolism/pathology
Caspase 3/metabolism
Caspase 9/metabolism
Cell Survival/drug effects
Flow Cytometry
Hep G2 Cells
Humans
Indoles/chemistry
Liver Neoplasms/*drug therapy/metabolism/pathology
Plant Bark/chemistry
Plant Extracts/*pharmacology
Poly(ADP-ribose) Polymerases/metabolism
Ulmus/*chemistry
bcl-2-Associated X Protein/metabolism
Figure
Reference
-
1. Abrams P, Marsh JW. Current approach to hepatocellular carcinoma. Surg Clin North Am. 2010. 90:803–816.
Article2. Choi SY, Lee S, Choi WH, Lee Y, Jo YO, Ha TY. Isolation and anti-inflammatory activity of bakuchiol from Ulmus davidiana var. Japonica. J Med Food. 2010. 13:1019–1023.
Article3. Costantini P, Jacotot E, Decaudin D, Kroemer G. Mitochondrion as a novel target of anticancer chemotherapy. J Natl Cancer Inst. 2000. 92:1042–1053.
Article4. Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999. 68:383–424.
Article5. Eun JS, Song WY. The combined effects of n-BuOH fraction of ulmi cortex and anticancer drugs on cancer cell lines. Korean J Pharmacogn. 1994. 25:144–152.6. Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001. 3:E255–E263.
Article7. Green D, Kroemer G. The central executioners of apoptosis: caspases or mitochondria? Trends Cell Biol. 1998. 8:267–271.
Article8. Henderson PJF, Lardy HA. Bongkrekic acid. An inhibitor of the adenine nucleotide translocase of mitochondria. J Biol Chem. 1970. 245:1319–1326.9. Hengartner MO. The biochemistry of apoptosis. Nature. 2000. 407:770–776.
Article10. Hill LL, Ouhtit A, Loughlin SM, Kripke ML, Ananthaswamy HN, Owen-Schaub LB. Fas ligand: a sensor for DNA damage critical in skin cancer etiology. Science. 1999. 285:898–900.
Article11. Jun CD, Pae HO, Kim YC, Jeong SJ, Yoo JC, Lee EJ, Choi BM, Chae SW, Park RK, Chung HT. Inhibition of nitric oxide synthesis by butanol fraction of the methanol extract of Ulmus davidiana in murine macrophages. J Ethnopharmacol. 1998. 62:129–135.
Article12. Jung MJ, Heo SI, Wang MH. Free radical scavenging and total phenolic contents from methanolic extracts of Ulmus davidiana. Food Chem. 2008. 108:482–487.
Article13. Kaiser GM, Sotiropoulos GC, Sgourakis G, Bleck J, Baba HA, Beckebaumr S, Gerken G, Paul A, Trarbach T. Surgical treatment of Klatskin tumor: liver resection versus transplantation. Hepatogastroenterology. 2010. 57:1337–1340.14. Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972. 26:239–257.
Article15. Kim JP, Kim WG, Koshino H, Jung J, Yoo ID. Sesquiterpene O-naphthoquinones from the root bark of Ulmus davidiana. Phytochemistry. 1996. 43:425–430.
Article16. Kohle C, Schwarz M, Bock KW. Promotion of hepatocarcinogenesis in humans and animal models. Arch Toxicol. 2008. 82:623–631.
Article17. Kroemer G, Petit P, Zamzami N, Vayssiere JL, Mignotte B. The biochemistry of programmed cell death. FASEB J. 1995. 9:1277–1287.
Article18. Kwon HJ, Kim TM, Ryu JM, Son SH, Hong JT, Jeong HS, Kang JS, Ahn JY, Kim SR, Ha TY, Kim DJ. Chemopreventive effects of elm tree root extract on colonic aberrant crypt foci induced by 1,2-dimethylhydrazine in F344 rats. J Food Sci Nutr. 2008. 13:157–165.
Article19. Lee DH, Kim JH, Nam JJ, Kim HR, Shin HR. Epidemiological findings of hepatitis B infection based on 1998 National Health and Nutrition Survey in Korea. J Korean Med Sci. 2002. 17:457–462.
Article20. Lee JC, Lim KT. Inhibitory effects of the ethanol extract of Ulmus davidiana on apoptosis induced by glucose-glucose oxidase and cytokine production in cultured mouse primary immune cells. J Biochem Mol Biol. 2001. 34:463–471.21. Lee KH, Cho CH, Yoon WH. In vivo antitumor activity of mansonone E isolated from Ulmus davidiana var. japonica Nakai. Korean J Pharmacogn. 2004. 35:199–202.22. Lee SJ, editor. Korean Folk Medicine. 1966. Seoul: Seoul National University;39. No. 3.23. Lee SJ, Lim KT. UDN glycoprotein regulates activities of manganese-superoxide dismutase, activator protein-1, and nuclear factor-kB stimulated by reactive oxygen radicals in lipopolysaccharide-stimulated HCT-116 cells. Cancer Lett. 2007. 254:274–287.
Article24. Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996. 86:147–157.
Article25. Quignon F, De Bels F, Koken M, Feunteun J, Ameisen JC, de Thé H. PML induces a novel caspase-independent death process. Nat Genet. 1998. 20:259–265.
Article26. Reed JC. Apoptosis-targeted therapies for cancer. Cancer Cell. 2003. 3:17–22.
Article27. Son BW, Park JH, Zee OP. Catechin glycoside from Ulmus davidiana. Arch Pharm Res. 1989. 12:219–222.28. Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999. 397:441–446.
Article29. Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998. 281:1312–1316.
Article30. Wang D, Xia M, Cui Z, Tashiro S, Onodera S, Ikejima T. Cytotoxic effects of mansonone E and F isolated from Ulmus pumila. Biol Pharm Bull. 2004. 27:1025–1030.31. Wild CP, Hall AJ. Primary prevention of hepatocellular carcinoma in developing countries. Mutat Res. 2000. 462:381–393.
Article32. Wolf BB, Green DR. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J Biol Chem. 1999. 274:20049–20052.
Article33. Wu M, Xu LG, Li X, Zhai Z, Shu HB. AMID, an apoptosis-inducing factor-homologous mitochondrion-associated protein, induces caspase-independent apoptosis. J Biol Chem. 2002. 277:25617–25623.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two cases of acute toxic hepatitis and renal failure after ingestion of the extract of elm bark in lung cancer patients
- Mariannaea samuelsii Isolated from a Bark Beetle-Infested Elm Tree in Korea
- The Apoptotic Effect of the Hexane Extract of Rheum undulatum L. in Oral Cancer Cells through the Down-regulation of Specificity Protein 1 and Survivin
- Differential Thioredoxin Reductase Activity from Human Normal Hepatic and Hepatoma Cell Lines
- A Case of Acute Toxic Hepatitis and Acute Kidney Injury after Ingestion of Ulmus davidiana var. japonica Extracts