J Korean Med Sci.
2011 Nov;26(11):1489-1494. 10.3346/jkms.2011.26.11.1489.
Apoptotic Effects of Genistein, Biochanin-A and Apigenin on LNCaP and PC-3 Cells by p21 through Transcriptional Inhibition of Polo-like Kinase-1
- Affiliations
-
- 1Department of Urology, College of Medicine, Dongguk University, Gyeongju, Korea.
- 2Department of Urology, School of Medicine, Kyungpook National University, Daegu, Korea. tgkwon@knu.ac.kr
- 3Joint Institute for Regenerative Medicine, Kyungpook National University Hospital, Daegu, Korea.
- 4Department of Pathology, School of Medicine, Kyungpook National University, Daegu, Korea.
- KMID: 1123450
- DOI: http://doi.org/10.3346/jkms.2011.26.11.1489
Abstract
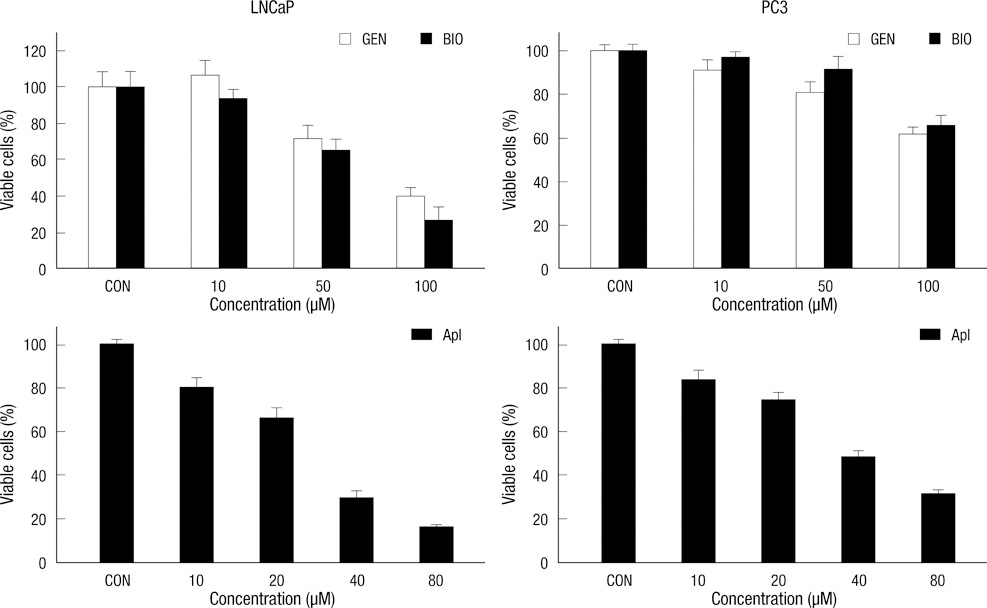
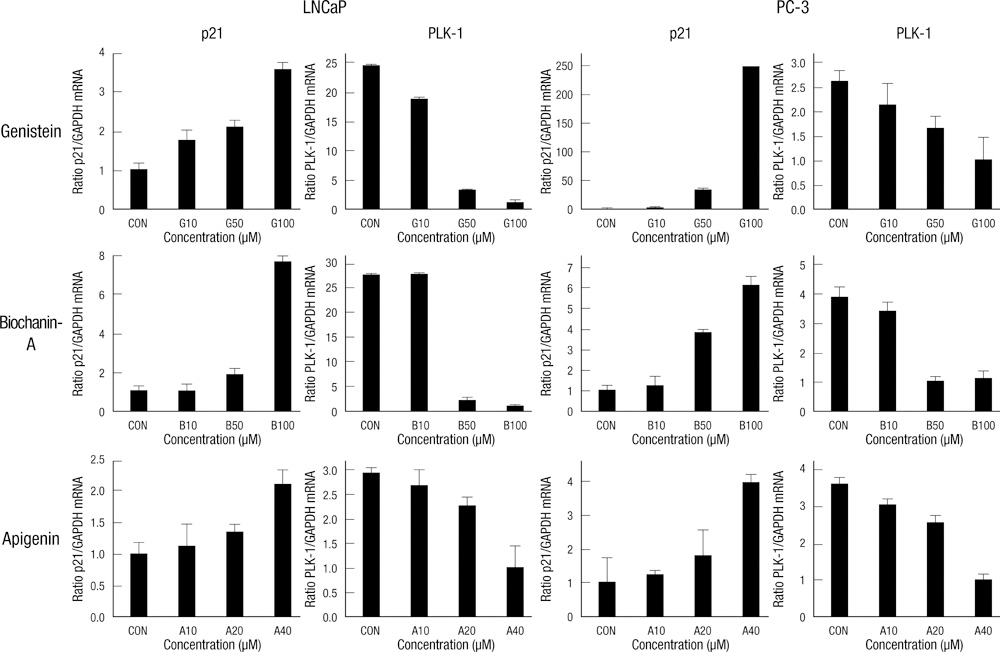
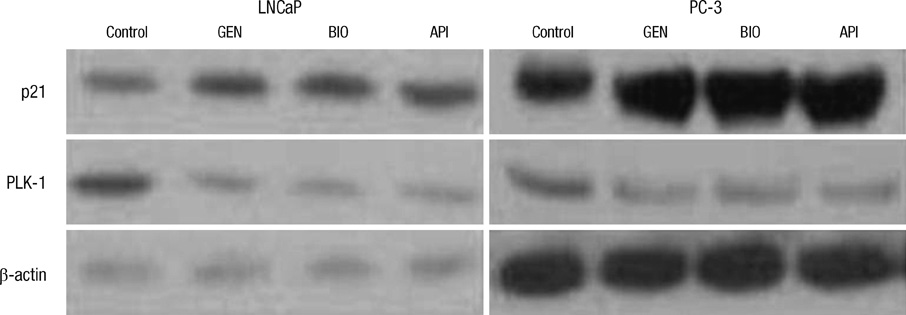
- Natural isoflavones and flavones are important dietary factors for prostate cancer prevention. We investigated the molecular mechanism of these compounds (genistein, biochanin-A and apigenin) in PC-3 (hormone-independent/p53 mutant type) and LNCaP (hormone-dependent/p53 wild type) prostate cancer cells. A cell growth rate and apoptotic activities were analyzed in different concentrations and exposure time to evaluate the antitumor activities of genistein, biochanin-A and apigenin. The real time PCR and Western blot analysis were performed to investigate whether the molecular mechanism of these compounds are involving the p21 and PLK-1 pathway. Apoptosis of prostate cancer cells was associated with p21 up-regulation and PLK-1 suppression. Exposure of genistein, biochanin-A and apigenin on LNCaP and PC-3 prostate cancer cells resulted in same pattern of cell cycle arrest and apoptosis. The inhibition effect for cell proliferation was slightly greater in LNCaP than PC-3 cells. In conclusion, flavonoids treatment induces up-regulation of p21 expression, and p21 inhibits transcription of PLK-1, which promotes apoptosis of cancer cells.
Keyword
MeSH Terms
-
Antineoplastic Agents/*pharmacology
Apigenin/pharmacology
*Apoptosis
Cell Cycle/drug effects
Cell Cycle Proteins/biosynthesis/*genetics/metabolism
Cell Line, Tumor
Cell Proliferation
Cyclin-Dependent Kinase Inhibitor p21/biosynthesis/*metabolism
Flavonoids/*pharmacology
Gene Expression Regulation, Neoplastic/drug effects
Genistein/pharmacology
Humans
Male
Prostatic Neoplasms/genetics/metabolism/*pathology
Protein Kinase Inhibitors/pharmacology
Protein-Serine-Threonine Kinases/biosynthesis/*genetics/metabolism
Proto-Oncogene Proteins/biosynthesis/*genetics/metabolism
Transcription, Genetic/drug effects
Figure
Reference
-
1. Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003. 53:5–26.2. Trojan L, Kiknavelidze K, Knoll T, Alken P, Michel MS. Prostate cancer therapy: standard management, new options and experimental approaches. Anticancer Res. 2005. 25:551–561.3. Kucuk O. Chemoprevention of prostate cancer. Cancer Metastasis Rev. 2002. 21:111–124.4. Boué SM, Carter-Wientjes CH, Shih BY, Cleveland TE. Identification of flavone aglycones and glycosides in soybean pods by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2003. 991:61–68.5. Morito K, Hirose T, Kinjo J, Hirakawa T, Okawa M, Nohara T, Ogawa S, Inoue S, Muramatsu M, Masamune Y. Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol Pharm Bull. 2001. 24:351–356.6. Ying C, Hsu JT, Hung HC, Lin DH, Chen LF, Wang LK. Growth and cell cycle regulation by isoflavones in human breast carcinoma cells. Reprod Nutr Dev. 2002. 42:55–64.7. Medjakovic S, Jungbauer A. Red clover isoflavones biochanin A and formononetin are potent ligands of the human aryl hydrocarbon receptor. J Steroid Biochem Mol Biol. 2008. 108:171–177.8. Lindenmeyer F, Li H, Menashi S, Soria C, Lu H. Apigenin acts on the tumor cell invasion process and regulates protease production. Nutr Cancer. 2001. 39:139–147.9. Ujiki MB, Ding XZ, Salabat MR, Bentrem DJ, Golkar L, Milam B, Talamonti MS, Bell RH Jr, Iwamura T, Adrian TE. Apigenin inhibits pancreatic cancer cell proliferation through G2/M cell cycle arrest. Mol Cancer. 2006. 5:76.10. Wang W, Heideman L, Chung CS, Pelling JC, Koehler KJ, Birt DF. Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol Carcinog. 2000. 28:102–110.11. Dai W, Cogswell JP. Polo-like kinases and the microtubule organization center: targets for cancer therapies. Prog Cell Cycle Res. 2003. 5:327–334.12. Ahmad N. Polo-like kinase (Plk) 1: a novel target for the treatment of prostate cancer. FASEB J. 2004. 18:5–7.13. Pesin JA, Orr-Weaver TL. Regulation of APC/C activators in mitosis and meiosis. Annu Rev Cell Dev Biol. 2008. 24:475–499.14. Jalili A, Moser A, Pashenkov M, Wagner C, Pathria G, Borgdorff V, Gschaider M, Stingl G, Ramaswamy S, Wagner SN. Polo-like kinase 1 is a potential therapeutic target in human melanoma. J Invest Dermatol. 2011. 131:1886–1895.15. McKenzie L, King S, Marcar L, Nicol S, Dias SS, Schumm K, Robertson P, Bourdon JC, Perkins N, Fuller-Pace F, Meek DW. p53-dependent repression of polo-like kinase-1 (PLK1). Cell Cycle. 2010. 9:4200–4212.16. Archambault V, Glover DM. Polo-like kinases: conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol. 2009. 10:265–275.17. Reagan-Shaw S, Ahmad N. Silencing of polo-like kinase (Plk) 1 via siRNA causes induction of apoptosis and impairment of mitosis machinery in human prostate cancer cells: implications for the treatment of prostate cancer. FASEB J. 2005. 19:611–613.18. Malumbres M, Barbacid M. Cell cycle kinases in cancer. Curr Opin Genet Dev. 2007. 17:60–65.19. Ando K, Ozaki T, Yamamoto H, Furuya K, Hosoda M, Hayashi S, Fukuzawa M, Nakagawara A. Polo-like kinase 1 (Plk1) inhibits p53 function by physical interaction and phosphorylation. J Biol Chem. 2004. 279:25549–25561.20. Salvatore G, Nappi TC, Salerno P, Jiang Y, Garbi C, Ugolini C, Miccoli P, Basolo F, Castellone MD, Cirafici AM, Melillo RM, Fusco A, Bittner ML, Santoro M. A cell proliferation and chromosomal instability signature in anaplastic thyroid carcinoma. Cancer Res. 2007. 67:10148–10158.21. Kreis NN, Sommer K, Sanhaji M, Kramer A, Matthess Y, Kaufmann M, Strebhardt K, Yuan J. Long-term downregulation of Polo-like kinase 1 increases the cyclin-dependent kinase inhibitor p21(WAF1/CIP1). Cell Cycle. 2009. 8:460–472.22. Lei M, Erikson RL. Plk1 depletion in nontransformed diploid cells activates the DNA-damage checkpoint. Oncogene. 2008. 27:3935–3943.23. Ismail IA, Kang KS, Lee HA, Kim JW, Sohn YK. Genistein-induced neuronal apoptosis and G2/M cell cycle arrest is associated with MDC1 up-regulation and PLK1 down-regulation. Eur J Pharmacol. 2007. 575:12–20.24. Zhao R, Xiang N, Domann FE, Zhong W. Effects of selenite and genistein on G2/M cell cycle arrest and apoptosis in human prostate cancer cells. Nutr Cancer. 2009. 61:397–407.25. Shukla S, Gupta S. Apigenin-induced prostate cancer cell death is initiated by reactive oxygen species and p53 activation. Free Radic Biol Med. 2008. 44:1833–1845.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Melatonin Induces Apoptotic Cell Death via p53 in LNCaP Cells
- Genistein Induces G2/M Cell Cycle Arrest and Apoptosis in Rat Neuroblastoma B35 Cells; Involvement of p21(waf1/cip1), Bax and Bcl-2
- Molecular Mechanism of the G2/M Arrest in Breast Cancer Cell Lines (T47D and MDA-MB231) Induced by Genistein
- Preferential Cytotoxic Effect of Genistein on G361 Melanoma Cells Via Inhibition of the Expression of Focal Adhesion Kinase
- Role of p21CIP1 as a determinant of SC-560 response in human HCT116 colon carcinoma cells