Yonsei Med J.
2012 Mar;53(2):294-303. 10.3349/ymj.2012.53.2.294.
Effects of N-Acetylcysteine on Nicotinamide Dinucleotide Phosphate Oxidase Activation and Antioxidant Status in Heart, Lung, Liver and Kidney in Streptozotocin-Induced Diabetic Rats
- Affiliations
-
- 1Department of Pharmacology, School of Basic Medical Science, Wuhan University, Wuhan, China. zhengyuan_xia@yahoo.com
- 2Department of Anesthesiology, The University of Hong Kong, Hong Kong SAR, China.
- 3Department of Biochemistry and Molecular Biology, School of Basic Medical Science, Wuhan University, Wuhan, China.
- KMID: 1120195
- DOI: http://doi.org/10.3349/ymj.2012.53.2.294
Abstract
- PURPOSE
Hyperglycemia increases reactive oxygen species (ROS) and the resulting oxidative stress plays a key role in the pathogenesis of diabetic complications. Nicotinamide dinucleotide phosphate (NADPH) oxidase is one of the major sources of ROS production in diabetes. We, therefore, examined the possibility that NADPH oxidase activation is increased in various tissues, and that the antioxidant N-acetylcysteine (NAC) may have tissue specific effects on NADPH oxidase and tissue antioxidant status in diabetes.
MATERIALS AND METHODS
Control (C) and streptozotocin-induced diabetic (D) rats were treated either with NAC (1.5 g/kg/day) orally or placebo for 4 weeks. The plasma, heart, lung, liver, kidney were harvested immediately and stored for biochemical or immunoblot analysis.
RESULTS
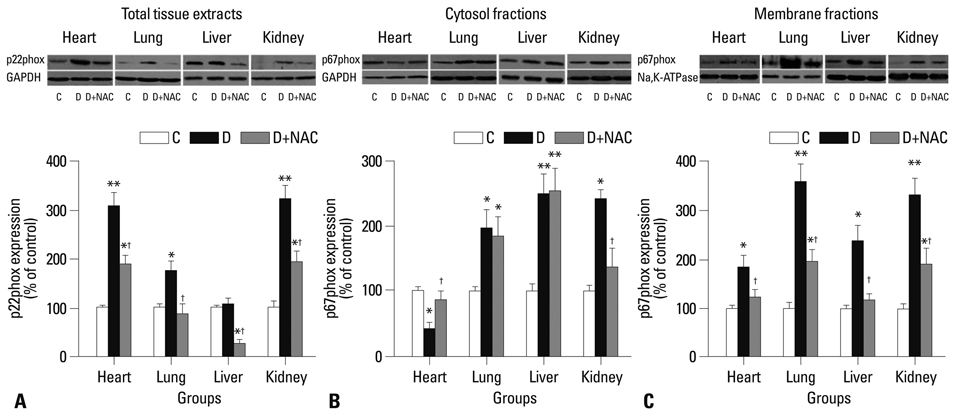
levels of free 15-F2t-isoprostane were increased in plasma, heart, lung, liver and kidney tissues in diabetic rats, accompanied with significantly increased membrane translocation of the NADPH oxidase subunit p67phox in all tissues and increased expression of the membrane-bound subunit p22phox in heart, lung and kidney. The tissue antioxidant activity in lung, liver and kidney was decreased in diabetic rats, while it was increased in heart tissue. NAC reduced the expression of p22phox and p67phox, suppressed p67phox membrane translocation, and reduced free 15-F(2t)-isoprostane levels in all tissues. NAC increased antioxidant activity in liver and lung, but did not significantly affect antioxidant activity in heart and kidney.
CONCLUSION
The current study shows that NAC inhibits NADPH oxidase activation in diabetes and attenuates tissue oxidative damage in all organs, even though its effects on antioxidant activity are tissue specific.
MeSH Terms
Figure
Reference
-
1. Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010. 362:1090–1101.
Article2. Hakim FA, Pflueger A. Role of oxidative stress in diabetic kidney disease. Med Sci Monit. 2010. 16:RA37–RA48.3. Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999. 48:1–9.
Article4. Ceriello A, Testa R. Antioxidant anti-inflammatory treatment in type 2 diabetes. Diabetes Care. 2009. 32:Suppl 2. S232–S236.
Article5. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001. 414:813–820.
Article6. Robinson I, de Serna DG, Gutierrez A, Schade DS. Vitamin E in humans: an explanation of clinical trial failure. Endocr Pract. 2006. 12:576–582.
Article7. Hasnain BI, Mooradian AD. Recent trials of antioxidant therapy: what should we be telling our patients? Cleve Clin J Med. 2004. 71:327–334.
Article8. Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009. 106:8665–8670.
Article9. Gao L, Mann GE. Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc Res. 2009. 82:9–20.
Article10. Shen GX. Oxidative stress and diabetic cardiovascular disorders: roles of mitochondria and NADPH oxidase. Can J Physiol Pharmacol. 2010. 88:241–248.
Article11. Lyle AN, Griendling KK. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology (Bethesda). 2006. 21:269–280.
Article12. Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000. 86:494–501.13. Liu S, Ma X, Gong M, Shi L, Lincoln T, Wang S. Glucose down-regulation of cGMP-dependent protein kinase I expression in vascular smooth muscle cells involves NAD(P)H oxidase-derived reactive oxygen species. Free Radic Biol Med. 2007. 42:852–863.
Article14. Li L, Renier G. Activation of nicotinamide adenine dinucleotide phosphate (reduced form) oxidase by advanced glycation end products links oxidative stress to altered retinal vascular endothelial growth factor expression. Metabolism. 2006. 55:1516–1523.
Article15. Picchi A, Gao X, Belmadani S, Potter BJ, Focardi M, Chilian WM, et al. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ Res. 2006. 99:69–77.
Article16. Guo Z, Xia Z, Jiang J, McNeill JH. Downregulation of NADPH oxidase, antioxidant enzymes, and inflammatory markers in the heart of streptozotocin-induced diabetic rats by N-acetyl-L-cysteine. Am J Physiol Heart Circ Physiol. 2007. 292:H1728–H1736.17. Sandström J, Nilsson P, Karlsson K, Marklund SL. 10-fold increase in human plasma extracellular superoxide dismutase content caused by a mutation in heparin-binding domain. J Biol Chem. 1994. 269:19163–19166.
Article18. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005. 54:1615–1625.19. Kowluru RA, Atasi L, Ho YS. Role of mitochondrial superoxide dismutase in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2006. 47:1594–1599.
Article20. Madsen-Bouterse SA, Zhong Q, Mohammad G, Ho YS, Kowluru RA. Oxidative damage of mitochondrial DNA in diabetes and its protection by manganese superoxide dismutase. Free Radic Res. 2010. 44:313–321.
Article21. Reis JS, Bosco AA, Veloso CA, Mattos RT, Purish S, Nogueira-Machado JA. Oxidizing and reducing responses in type 1 diabetic patients determined up to 5 years after the clinical onset of the disease. Acta Diabetol. 2008. 45:221–224.
Article22. Ivanović-Matić S, Mihailović M, Dinić S, Martinović V, Bogojević D, Grigorov I, et al. The absence of cardiomyopathy is accompanied by increased activities of CAT, MnSOD and GST in long-term diabetes in rats. J Physiol Sci. 2010. 60:259–266.
Article23. Ghosh S, Pulinilkunnil T, Yuen G, Kewalramani G, An D, Qi D, et al. Cardiomyocyte apoptosis induced by short-term diabetes requires mitochondrial GSH depletion. Am J Physiol Heart Circ Physiol. 2005. 289:H768–H776.
Article24. Yue KK, Chung WS, Leung AW, Cheng CH. Redox changes precede the occurrence of oxidative stress in eyes and aorta, but not in kidneys of diabetic rats. Life Sci. 2003. 73:2557–2570.
Article25. Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989. 6:593–597.
Article26. Xia Z, Kuo KH, Nagareddy PR, Wang F, Guo Z, Guo T, et al. N-acetylcysteine attenuates PKCbeta2 overexpression and myocardial hypertrophy in streptozotocin-induced diabetic rats. Cardiovasc Res. 2007. 73:770–782.
Article27. Meng X, Tancharoen S, Kawahara KI, Nawa Y, Taniguchi S, Hashiguchi T, et al. 1,5-Anhydroglucitol attenuates cytokine release and protects mice with type 2 diabetes from inflammatory reactions. Int J Immunopathol Pharmacol. 2010. 23:105–119.
Article28. Xu M, Dai DZ, Zhang Q, Cheng YS, Dai Y. Upregulated NADPH oxidase contributes to diabetic testicular complication and is relieved by strontium fructose 1,6-diphosphate. Exp Clin Endocrinol Diabetes. 2010. 118:459–465.
Article29. Wu HC, Wang Q, Yang HI, Ahsan H, Tsai WY, Wang LY, et al. Urinary 15-F2t-isoprostane, aflatoxin B1 exposure and hepatitis B virus infection and hepatocellular carcinoma in Taiwan. Carcinogenesis. 2008. 29:971–976.
Article30. Faure P, Polge C, Monneret D, Favier A, Halimi S. Plasma 15-F2t isoprostane concentrations are increased during acute fructose loading in type 2 diabetes. Diabetes Metab. 2008. 34:148–154.
Article31. Mandal CC, Ganapathy S, Gorin Y, Mahadev K, Block K, Abboud HE, et al. Reactive oxygen species derived from Nox4 mediate BMP2 gene transcription and osteoblast differentiation. Biochem J. 2011. 433:393–402.
Article32. Wold LE, Ceylan-Isik AF, Ren J. Oxidative stress and stress signaling: menace of diabetic cardiomyopathy. Acta Pharmacol Sin. 2005. 26:908–917.
Article33. Du D, Shi YH, Le GW. Oxidative stress induced by high-glucose diet in liver of C57BL/6J mice and its underlying mechanism. Mol Biol Rep. 2010. 37:3833–3839.
Article34. Ahmed FN, Naqvi FN, Shafiq F. Lipid peroxidation and serum antioxidant enzymes in patients with type 2 diabetes mellitus. Ann N Y Acad Sci. 2006. 1084:481–489.
Article35. Ramakrishna V, Jailkhani R. Oxidative stress in non-insulin-dependent diabetes mellitus (NIDDM) patients. Acta Diabetol. 2008. 45:41–46.
Article36. Hünkar T, Aktan F, Ceylan A, Karasu C. Antioxidants in Diabetes-Induced Complications (ADIC) Study Group. Effects of cod liver oil on tissue antioxidant pathways in normal and streptozotocin-diabetic rats. Cell Biochem Funct. 2002. 20:297–302.
Article37. Olukman M, Orhan CE, Celenk FG, Ulker S. Apocynin restores endothelial dysfunction in streptozotocin diabetic rats through regulation of nitric oxide synthase and NADPH oxidase expressions. J Diabetes Complications. 2010. 24:415–423.
Article38. Lenaz G, Bovina C, D'Aurelio M, Fato R, Formiggini G, Genova ML, et al. Role of mitochondria in oxidative stress and aging. Ann N Y Acad Sci. 2002. 959:199–213.
Article39. Fosslien E. Mitochondrial medicine--molecular pathology of defective oxidative phosphorylation. Ann Clin Lab Sci. 2001. 31:25–67.40. Sack MN. Type 2 diabetes, mitochondrial biology and the heart. J Mol Cell Cardiol. 2009. 46:842–849.
Article41. Kowluru RA, Kowluru V, Xiong Y, Ho YS. Overexpression of mitochondrial superoxide dismutase in mice protects the retina from diabetes-induced oxidative stress. Free Radic Biol Med. 2006. 41:1191–1196.
Article42. Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010. 140:900–917.
Article43. Casas-Agustench P, Bulló M, Salas-Salvadó J. Nuts, inflammation and insulin resistance. Asia Pac J Clin Nutr. 2010. 19:124–130.44. Navarro-Gonzalez J, Mora-Fernandez C, Gomez-Chinchon M, Muros M, Herrera H, Garcia J. Serum and gene expression profile of tumor necrosis factor-alpha and interleukin-6 in hypertensive diabetic patients: effect of amlodipine administration. Int J Immunopathol Pharmacol. 2010. 23:51–59.
Article45. Wu W, Wang M, Sun Z, Wang X, Miao J, Zheng Z. The predictive value of TNF-alpha and IL-6 and the incidence of macrovascular complications in patients with type 2 diabetes. Acta Diabetol. 2012. Epub ahead of print.46. Tsai GY, Cui JZ, Syed H, Xia Z, Ozerdem U, McNeill JH, et al. Effect of N-acetylcysteine on the early expression of inflammatory markers in the retina and plasma of diabetic rats. Clin Experiment Ophthalmol. 2009. 37:223–231.
Article47. Palacio JR, Markert UR, Martínez P. Anti-inflammatory properties of N-acetylcysteine on lipopolysaccharide-activated macrophages. Inflamm Res. 2011. 60:695–704.
Article48. Nascimento MM, Suliman ME, Silva M, Chinaglia T, Marchioro J, Hayashi SY, et al. Effect of oral N-acetylcysteine treatment on plasma inflammatory and oxidative stress markers in peritoneal dialysis patients: a placebo-controlled study. Perit Dial Int. 2010. 30:336–342.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of S-allylcysteine on Oxidative Stress in Streptozotocin-Induced Diabetic Rats
- Effects of Benincasa Hispida Seed Supplementation on Glycogen Status and Lipid Peroxidation in Streptozotocin-Induced Diabetic Rats
- Tetrahydrocurcumin Ameliorates Kidney Injury and High Systolic Blood Pressure in High-Fat Diet-Induced Type 2 Diabetic Mice
- Effect of Red Ginseng Extract on Lipid Peroxidation in Streptozotocin-induced Diabetic Rats
- Effects of soybean isoflavone extract on the plasma lipid profiles and antioxidant enzyme activity in streptozotocin-induced diabetic rats