Yonsei Med J.
2006 Apr;47(2):214-222. 10.3349/ymj.2006.47.2.214.
In Vitro and In Vivo Effect of Parathyroid Hormone Analogue (1-14) Containing alpha-amino-iso-butyric Acid Residue (Aib)1,3
- Affiliations
-
- 1Department of Internal Medicine, Endocrine Research College of Medicine, Korea. lsk@yumc.yonsei.ac.kr
- 2Department of Biochemistry, College of Science, Yonsei University, Seoul, Korea.
- KMID: 1110745
- DOI: http://doi.org/10.3349/ymj.2006.47.2.214
Abstract
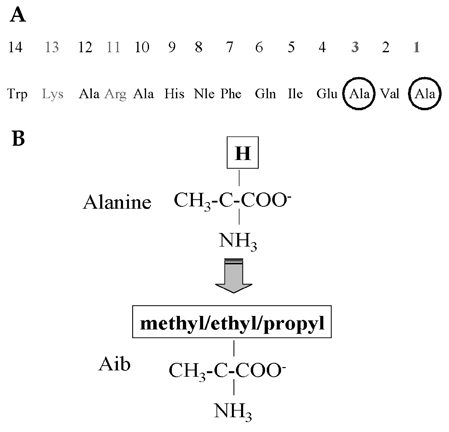
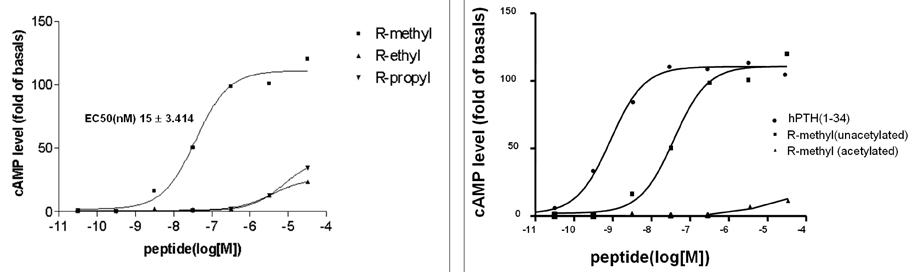
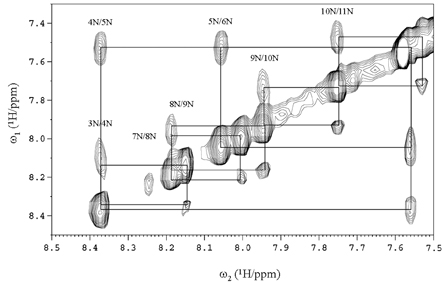
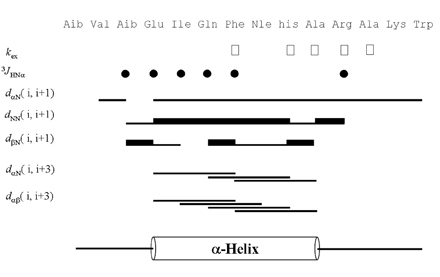
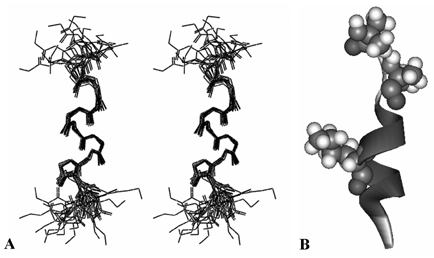
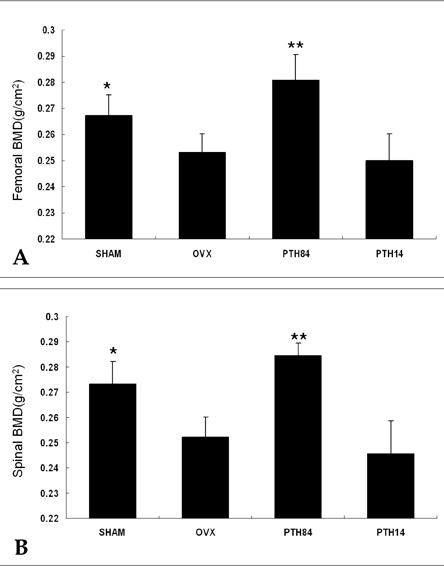
- Firstly, parathyroid hormone (1-14) [PTH (1-14)] analogue containing various alpha-amino-iso-butyric acid residue (Aib) was synthesized by exchanging the 1st and 3rd Ala residues of alpha carbon of PTH (1-14). This analogue revealed to have the quite tight and stable alpha-helical structure using the nuclear magnetic resonance (NMR) analysis. The biological activities of these analogues were examined using a cAMP-generating assay in LLC-PK1 cell lines stably transfected with the wild-type human PTH1 receptor. Only the PTH analogue substituted with methyl moiety without acetylation showed significant cAMP generating action with 15.0 +/- 3.414 of EC50. Then, we used an ovariectomized rat model system to compare the in vivo effects of parathyroid hormone analogue with that of PTH (1-84). Daily subcutaneous administration of the unacetylated Aib1,3PTH (1-14) for 5 weeks in 30 nM/kg subcutaneously with positive control group receiving PTH (1-84) with 8 nM/ kg were performed. However, there was no significant change in spinal or femoral bone mineral density assessed by dual x-ray absorptiometry (DXA) in the Aib1,3PTH (1-14) group where definite increase of these parameters shown in the PTH (1-84) group (p < 0.001). Assessment of bone strength was evaluated with no significant differences among all groups. It was quite disappointing to see the actual discrepancies between the result of significant pharmacokinetic potency and the in vivo clinical effect of the Aib1,3PTH (1-14). However, there are several limitations to mention, such as the short duration of treatment, matter of dosage, and insufficient effect of tight alpha-helical structures with absence of C-terminus. In conclusion, our findings suggest that unacetylated Aib1,3PTH (1-14) did not exhibit any anabolic effects at the bones of ovariectomized rats.
Keyword
MeSH Terms
-
Transfection
Time Factors
Structure-Activity Relationship
Stress, Mechanical
Spectrometry, X-Ray Emission
Rats
Protein Structure, Tertiary
Protein Structure, Secondary
Protein Conformation
Protein Binding
Peptides/chemistry
Parathyroid Hormone/*analogs & derivatives/chemistry/*metabolism
Molecular Sequence Data
Molecular Conformation
Models, Statistical
Models, Molecular
Magnetic Resonance Spectroscopy
LLC-PK1 Cells
Humans
Female
Dose-Response Relationship, Drug
Densitometry
Cyclic AMP/metabolism
Cell Line
Bone and Bones/metabolism
Bone Density
Biomechanics
Animals
Aminoisobutyric Acids/*metabolism
Amino Acid Sequence
Alanine/chemistry
Figure
Reference
-
1. Consensus development conference: prophylaxis and treatment of osteoporosis. Am J Med. 1991. 90:107–110.2. Alexander JM, Bab I, Fish S, Muller R, Uchiyama T, Gronowicz G, et al. Human parathyroid hormone 1-34 reverses bone loss in ovariectomized mice. J Bone Miner Res. 2001. 16:1665–1673.3. Burr DB, Hirano T, Turner CH, Hotchkiss C, Brommage R, Hock JM. Intermittently administered human parathyroid hormone(1-34) treatment increases intracortical bone turnover and porosity without reducing bone strength in the humerus of ovariectomized cynomolgus monkeys. J Bone Miner Res. 2001. 16:157–165.4. Kneissel M, Boyde A, Gasser JA. Bone tissue and its mineralization in aged estrogen-depleted rats after long-term intermittent treatment with parathyroid hormone (PTH) analog SDZ PTS 893 or human PTH (1-34). Bone. 2001. 28:237–250.5. Mashiba T, Burr DB, Turner CH, Sato M, Cain RL, Hock JM. Effects of human parathyroid hormone (1-34), LY333334, on bone mass, remodeling, and mechanical properties of cortical bone during the first remodeling cycle in rabbits. Bone. 2001. 28:538–547.6. Sato M, Zeng GQ, Turner CH. Biosynthetic human parathyroid hormone (1-34) effects on bone quality in aged ovariectomized rats. Endocrinology. 1997. 138:4330–4337.7. Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001. 344:1434–1441.8. Kronenberg HM, Lanske B, Kovacs CS, Chung UI, Lee K, Segre GV, et al. Functional analysis of the PTH/PTHrP network of ligands and receptors. Recent Prog Horm Res. 1998. 53:283–301. discussion 301-3.9. Armamento-Villareal R, Ziambaras K, Abbasi-Jarhomi SH, Dimarogonas A, Halstead L, Fausto A, et al. An intact N terminus is required for the anabolic action of parathyroid hormone on adult female rats. J Bone Miner Res. 1997. 12:384–392.10. Hilliker S, Wergedal JE, Gruber HE, Bettica P, Baylink DJ. Truncation of the amino terminus of PTH alters its anabolic activity on bone in vivo. Bone. 1996. 19:469–477.11. Takasu H, Gardella TJ, Luck MD, Potts JT Jr, Bringhurst FR. Amino-terminal modifications of human parathyroid hormone (PTH) selectively alter phospholipase C signaling via the type 1 PTH receptor: implications for design of signal-specific PTH ligands. Biochemistry. 1999. 38:13453–13460.12. Juppner H, Schipani E, Bringhurst FR, McClure I, Keutmann HT, Potts JT Jr, et al. The extracellular amino-terminal region of the parathyroid hormone (PTH)/PTH-related peptide receptor determines the binding affinity for carboxyl-terminal fragments of PTH-(1-34). Endocrinology. 1994. 134:879–884.13. Shimizu M, Potts JT Jr, Gardella TJ. Minimization of parathyroid hormone. Novel amino-terminal parathyroid hormone fragments with enhanced potency in activating the type-1 parathyroid hormone receptor. J Biol Chem. 2000. 275:21836–21843.14. Piserchio A, Bisello A, Rosenblatt M, Chorev M, Mierke DF. Characterization of parathyroid hormone/receptor interactions: structure of the first extracellular loop. Biochemistry. 2000. 39:8153–8160.15. Luck MD, Carter PH, Gardella TJ. The (1-14) fragment of parathyroid hormone (PTH) activates intact and amino-terminally truncated PTH-1 receptors. Mol Endocrinol. 1999. 13:670–680.16. Lee EJ, Kim HY, Cho MK, Lee W, Lim SK. Structure and function of a minimal receptor activation domain of parathyroid hormone. Yonsei Med J. 2004. 45:419–427.17. Davis DG, Bax A. Assignment of Complex 'H NMR Spectra via Two-Dimensional Homonuclear Hartmann-Hahn Spectroscopy. J Am Chem Soc. 1985. 107:2820–2821.18. Jeener J, Meier BH, Bachman P, Ernst RR. Investigation of exchange processes by two-dimensional NMR spectroscopy. J Chem Phys. 1979. 71:4546–4553.19. Rance M, Sorensen OW, Bodenhausen G, Wagner G, Ernst RR, Wuthrich K. Improved spectral resolution in cosy 1H NMR spectra of proteins via double quantum filtering. Biochem Biophys Res Commun. 1983. 117:479–485.20. Driscoll PC, Gronenborn AM, Beress L, Clore GM. Determination of the three-dimensional solution structure of the antihypertensive and antiviral protein BDS-I from the sea anemone Anemonia sulcata: a study using nuclear magnetic resonance and hybrid distance geometry-dynamical simulated annealing. Biochemistry. 1989. 28:2188–2198.21. Nilges M, Clore GM, Gronenborn AM. Determination of three-dimensional structures of proteins from interproton distance data by hybrid distance geometry-dynamical simulated annealing calculations. FEBS Lett. 1988. 229:317–324.22. Nilges M, Clore GM, Gronenborn AM. Determination of three-dimensional structures of proteins from interproton distance data by dynamical simulated annealing from a random array of atoms. Circumventing problems associated with folding. FEBS Lett. 1988. 239:129–136.23. Nilges M, Gronenborn AM, Brunger AT, Clore GM. Determination of three-dimensional structures of proteins by simulated annealing with interproton distance restraints. Application to crambin, potato carboxypeptidase inhibitor and barley serine proteinase inhibitor 2. Protein Eng. 1988. 2:27–38.24. Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 1998. 54:905–921.25. Wuthrich K. NMR proteins and Nucleic Acids. 1986. New York: Wiley.26. Karle IL, Balaram P. Structural characteristics of alpha-helical peptide molecules containing Aib residues. Biochemistry. 1990. 29:6747–6756.27. Bolin KA, Anderson DJ, Trulson JA, Thompson DA, Wilken J, Kent SB, et al. NMR structure of a minimized human agouti related protein prepared by total chemical synthesis. FEBS Lett. 1999. 451:125–131.28. Whitfield JF, Morley P, Willick G, MacLean S, Ross V, Isaacs RJ, et al. Comparison of the abilities of human parathyroid hormone (hPTH)-(1-34) and [Leu27]-cyclo (Glu22-Lys26)-hPTH-(1-31)NH2 to stimulate femoral trabecular bone growth in ovariectomized rats. Calcif Tissue Int. 1998. 63:423–428.29. Mohan S, Kutilek S, Zhang C, Shen HG, Kodama Y, Srivastava AK, et al. Comparison of bone formation responses to parathyroid hormone(1-34), (1-31), and (2-34) in mice. Bone. 2000. 27:471–478.30. Shimizu N, Petroni BD, Khatri A, Gardella TJ. Functional evidence for an intramolecular side chain interaction between residues 6 and 10 of receptor-bound parathyroid hormone analogues. Biochemistry. 2003. 42:2282–2290.31. Tsomaia N, Pellegrini M, Hyde K, Gardella TJ, Mierke DF. Toward parathyroid hormone minimization: conformational studies of cyclic PTH(1-14) analogues. Biochemistry. 2004. 43:690–699.32. Condon SM, Morize I, Darnbrough S, Burns CJ, Miller BE, Uhl J, et al. The bioactive conformation of human parathyroid hormone. Structural evidence for the extended helix postulate. J Am Chem Soc. 2000. 122:3007–3014.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Structure and Function of a Minimal Receptor Activation Domain of Parathyroid Hormone
- Comparative Analysis of Dibutyric cAMP and Butyric Acid on the Differentiation of Human Eosinophilic Leukemia EoL-1 Cells
- Adverse Side Effect of Baclofen: Case Report
- Alterations of Amino Acid Level in Depressed Rat Brain
- The Differentiation of HL-60 Cells Causes to Lose their Ability to Express TNF mRNA