Radioprotective effects of an acidic polysaccharide of Panax ginseng on bone marrow cells
- Affiliations
-
- 1Department of Veterinary Medicine, College of Applied Life Sciences, Cheju National University, Jeju 690-756, Korea. jooh@cheju.ac.kr
- 2Applied Radiological Science Research Institute, Cheju National University, Jeju 690-756, Korea.
- 3Department of Nuclear and Energy Engineering, College of Engineering, Cheju National University, Jeju 690-756, Korea.
- KMID: 1104234
- DOI: http://doi.org/10.4142/jvs.2007.8.1.39
Abstract
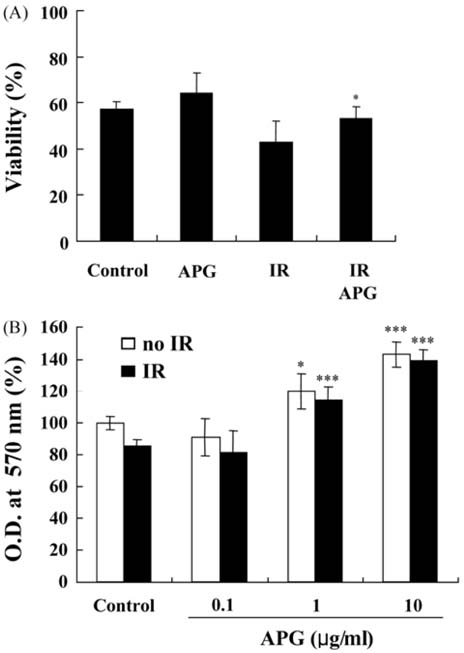
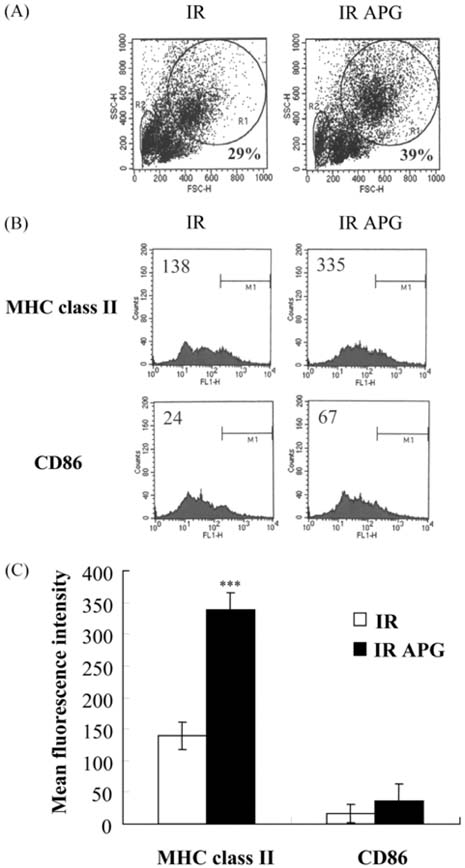
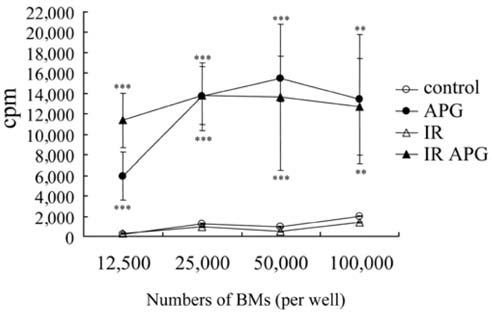
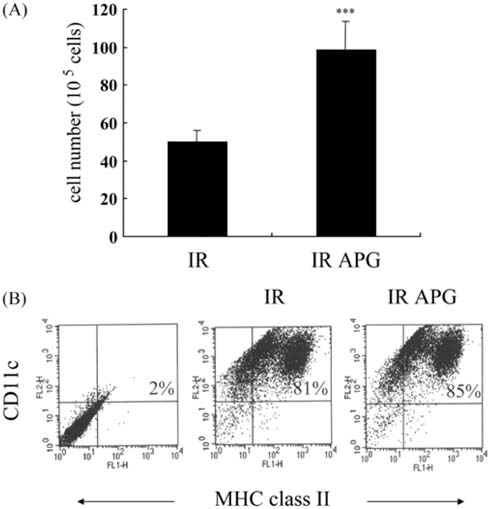
- An acidic polysaccharide of Panax ginseng (APG), so called ginsan is known to have important immunomodulatory activities. It was recently reported that APG has radioprotective effects in mice but the detailed mechanism was not fully elucidated. This study examined the effects of APG on bone marrow cells (BMs). The phenotypical and functional changes in APG-treated BMs after gamma radiation were studied. The benefit of APG on BMs damaged by gamma radiation was determined by measuring the cell viability. Using 2 different assays, a pretreatment with APG significantly increased the viability of BMs against gamma radiation. APG-treated BMs had a significantly higher amount of IL-12, which is a major cytokine for immune responses, compared with the medium-treated BMs. The expression of MHC class II molecules of APG-treated BMs was also increased, and APG-treated BMs showed significantly higher levels of allogeneic CD4+ T lymphocyte proliferation. Furthermore, APG-treated mice had a larger number of BMs after gamma radiation than the control mice, and the BMs of APG-treated mice were successfully cultured into dendritic cells, which are the representative antigenpresenting cells. Overall, this study shows that APG alters the phenotype of BMs, increases the viability and alloreactivity of BMs after gamma radiation both in vitro and in vivo. Therefore, APG may be a good candidate radioprotective agent for BMs.
MeSH Terms
-
Animals
Bone Marrow Cells/*drug effects/radiation effects
CD4-Positive T-Lymphocytes/metabolism
Cell Survival/radiation effects
Flow Cytometry
Gamma Rays
Interleukin-12/biosynthesis
Mice
Mice, Inbred BALB C
Mice, Inbred C57BL
Nitric Oxide/biosynthesis
Panax/*chemistry
Polysaccharides/*pharmacology
Radiation-Protective Agents/*pharmacology
Figure
Cited by 5 articles
-
The role of Bcl-xL and nuclear factor-κB in the effect of taxol on the viability of dendritic cells
Mi-Hyoung Kim, Hong-Gu Joo
J Vet Sci. 2009;10(2):99-103. doi: 10.4142/jvs.2009.10.2.99.Paclitaxel inhibits the hyper-activation of spleen cells by lipopolysaccharide and induces cell death
Hyun-Ji Kim, Hong-Gu Joo
J Vet Sci. 2016;17(4):453-458. doi: 10.4142/jvs.2016.17.4.453.Dapsone modulates lipopolysaccharide-activated bone marrow cells by inducing cell death and down-regulating tumor necrosis factor-α production
Min-Ji Kwon, Hong-Gu Joo
J Vet Sci. 2018;19(6):744-749. doi: 10.4142/jvs.2018.19.6.744.Radioprotective effects of fucoidan on bone marrow cells: improvement of the cell survival and immunoreactivity
Yun-Young Byon, Mi-Hyoung Kim, Eun-Sook Yoo, Kyu-Kye Hwang, Youngheun Jee, Taekyun Shin, Hong-Gu Joo
J Vet Sci. 2008;9(4):359-365. doi: 10.4142/jvs.2008.9.4.359.Immunomodulatory Activity of Ginsan, a Polysaccharide of
Panax Ginseng , on Dendritic Cells
Mi-Hyoung Kim, Yun-Young Byon, Eun-Ju Ko, Jie-Young Song, Yeon-Sook Yun, Taekyun Shin, Hong-Gu Joo
Korean J Physiol Pharmacol. 2009;13(3):169-173. doi: 10.4196/kjpp.2009.13.3.169.
Reference
-
1. Ahn JY, Choi IS, Shim JY, Yun EK, Yun YS, Jeong G, Song JY. The immunomodulator ginsan induces resistance to experimental sepsis by inhibiting Toll-like receptor-mediated inflammatory signals. Eur J Immunol. 2006. 36:37–45.
Article2. Arora R, Gupta D, Chawla R, Sagar R, Sharma A, Kumar R, Prasad J, Singh S, Samanta N, Sharma RK. Radioprotection by plant products: present status and future prospects. Phytother Res. 2005. 19:1–22.
Article3. Dalmau SR, Freitas CS, Savino W. Radio- and chemoprotection of bone marrow cells by opposite cell cycle-acting cytokines. Leuk Res. 1997. 21:93–99.
Article4. Han SK, Song JY, Yun YS, Yi SY. Ginsan improved Th1 immune response inhibited by gamma radiation. Arch Pharm Res. 2005. 28:343–350.
Article5. Han Y, Son SJ, Akhalaia M, Platonov A, Son HJ, Lee KH, Yun YS, Song JY. Modulation of radiation-induced disturbances of antioxidant defense systems by ginsan. Evid Based Complement Alternat Med. 2005. 2:529–536.
Article6. Ivanova T, Han Y, Son HJ, Yun YS, Song JY. Antimutagenic effect of polysaccharide ginsan extracted from Panax ginseng. Food Chem Toxicol. 2006. 44:517–521.
Article7. Joo HG, Goedegebuure PS, Sadanaga N, Nagoshi M, von Bernstorff W, Eberlein TJ. Expression and function of galectin-3, a beta-galactoside-binding protein in activated T lymphocytes. J Leukoc Biol. 2001. 69:555–564.8. Joo SS, Won TJ, Kim MS, Lee DI. Hematopoietic effect of ginsenoside Rg3 in ICR mouse primary cultures and its application to a biological response modifier. Fitoterapia. 2004. 75:337–341.
Article9. Kang KA, Zhang R, Lee KH, Chae S, Kim BJ, Kwak YS, Park JW, Lee NH, Hyun JW. Protective effect of triphlorethol-A from Ecklonia cava against ionizing radiation in vitro. J Radiat Res (Tokyo). 2006. 47:61–68.
Article10. Kumar M, Sharma MK, Saxena PS, Kumar A. Radioprotective effect of Panax ginseng on the phosphatases and lipid peroxidation level in testes of Swiss albino mice. Biol Pharm Bull. 2003. 26:308–312.
Article11. Lee HJ, Kim SR, Kim JC, Kang CM, Lee YS, Jo SK, Kim TH, Jang JS, Nah SY, Kim SH. In Vivo radioprotective effect of Panax ginseng C.A. Meyer and identification of active ginsenosides. Phytother Res. 2006. 20:392–395.
Article12. Lee TK, Johnke RM, Allison RR, O'Brien KF, Dobbs LJ Jr. Radioprotective potential of ginseng. Mutagenesis. 2005. 20:237–243.
Article13. Lee YS, Chung IS, Lee IR, Kim KH, Hong WS, Yun YS. Activation of multiple effector pathways of immune system by the antineoplastic immunostimulator acidic polysaccharide ginsan isolated from Panax ginseng. Anticancer Res. 1997. 17:323–331.14. Mori M, Desaintes C. Gene expression in response to ionizing radiation: an overview of molecular features in hematopoietic cells. J Biol Regul Homeost Agents. 2004. 18:363–371.15. Neta R. Modulation with cytokines of radiation injury: suggested mechanisms of action. Environ Health Perspect. 1997. 105:Suppl 6. 1463–1465.
Article16. Rades D, Fehlauer F, Bajrovic A, Mahlmann B, Richter E, Alberti W. Serious adverse effects of amifostine during radiotherapy in head and neck cancer patients. Radiother Oncol. 2004. 70:261–264.
Article17. Santini V, Giles FJ. The potential of amifostine: from cytoprotectant to therapeutic agent. Haematologica. 1999. 84:1035–1042.18. Shin JY, Song JY, Yun YS, Yang HO, Rhee DK, Pyo S. Immunostimulating effects of acidic polysaccharides extract of Panax ginseng on macrophage function. Immunopharmacol Immunotoxicol. 2002. 24:469–482.
Article19. Song JY, Akhalaia M, Platonov A, Kim HD, Jung IS, Han YS, Yun YS. Effects of polysaccharide ginsan from Panax ginseng on liver function. Arch Pharm Res. 2004. 27:531–538.
Article20. Song JY, Han SK, Bae KG, Lim DS, Son SJ, Jung IS, Yi SY, Yun YS. Radioprotective effects of ginsan, an immunomodulator. Radiat Res. 2003. 159:768–774.
Article21. Wrembel-Wargocka J, Jablonska H, Chomiczewski K. Clinical use of amifostine (WR-2721) as a preparation protecting healthy tissues from the cytotoxic effects of chemotherapy and radiation therapy. Przegl Lek. 1996. 53:820–825.22. Zhang JS, Sigdestad CP, Gemmell MA, Grdina DJ. Modification of radiation response in mice by fractionated extracts of Panax ginseng. Radiat Res. 1987. 112:156–163.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Brief Introduction of Panax ginseng C.A. Meyer
- Pathologic Comparative Studies on the Protective Effects by Panax Ginseng and Panax Quinquefolium for Treating 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced Toxicity in Male Rats
- Lipids in Ginseng (Panax ginseng) and Their Analysis
- Inhibitory effect of red ginseng acidic polysaccharide from Korean red ginseng on phagocytic activity and intracellular replication of Brucella abortus in RAW 264.7 cells
- Radioprotective effects of fucoidan on bone marrow cells: improvement of the cell survival and immunoreactivity