Nat Prod Sci.
2018 Mar;24(1):1-12. 10.20307/nps.2018.24.1.1.
Lipids in Ginseng (Panax ginseng) and Their Analysis
- Affiliations
-
- 1College of Pharmacy, Chung-Ang University, Seoul 06974, Republic of Korea. hykychoi@cau.ac.kr
- 2Division of Forensic Toxicology and Chemistry, Seoul institute, National Forensic Service, Seoul 08036, Republic of Korea.
- KMID: 2409603
- DOI: http://doi.org/10.20307/nps.2018.24.1.1
Abstract
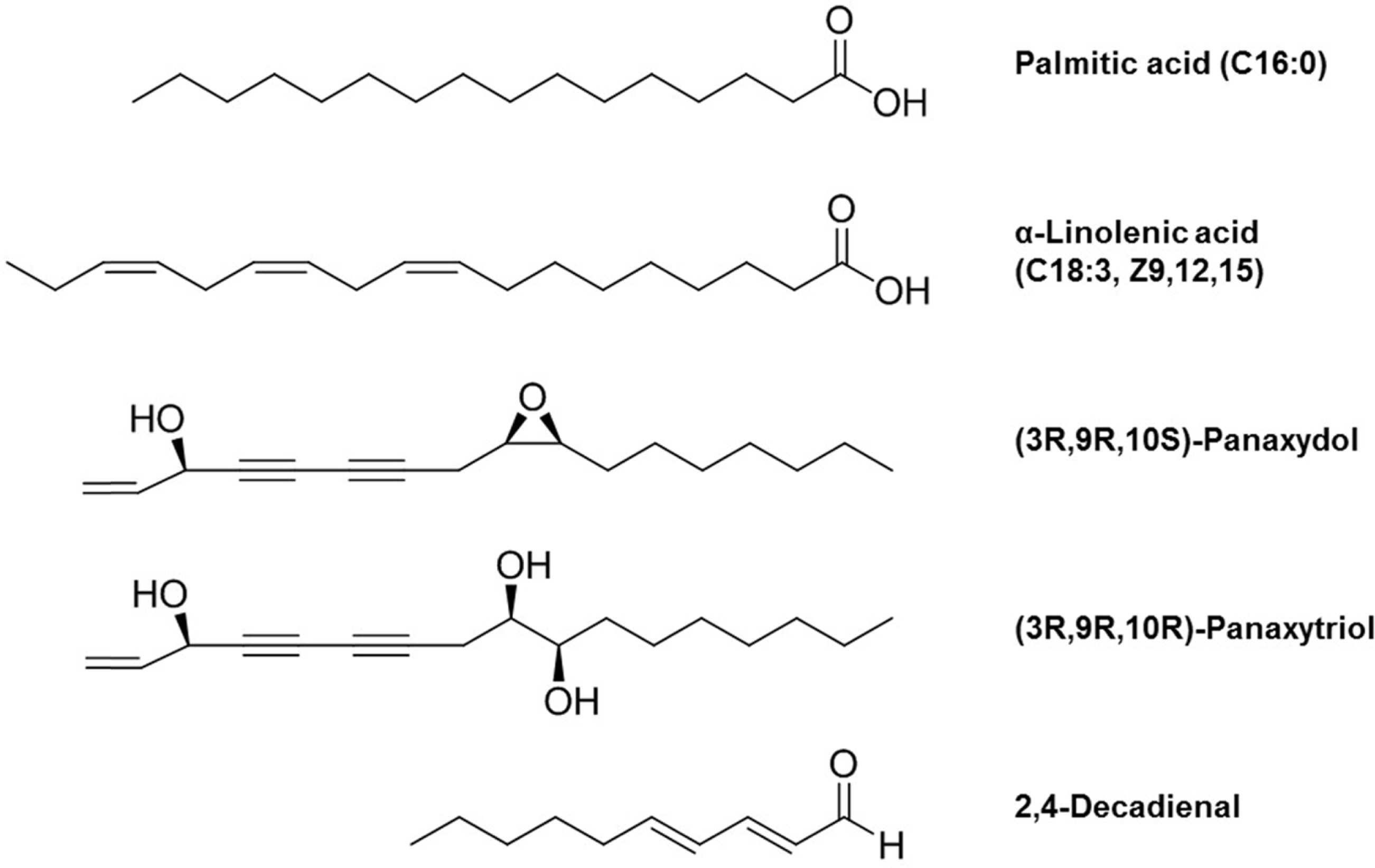
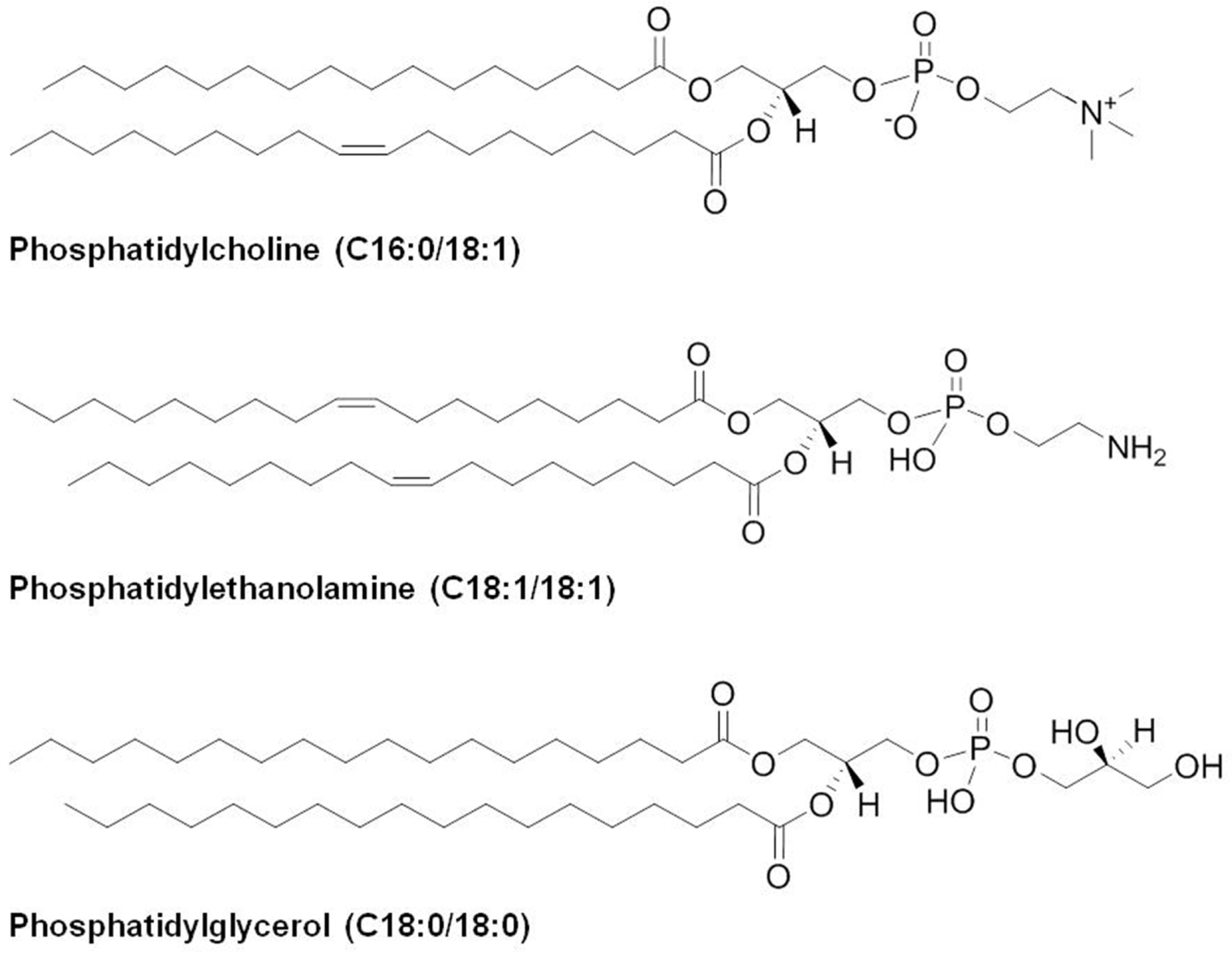
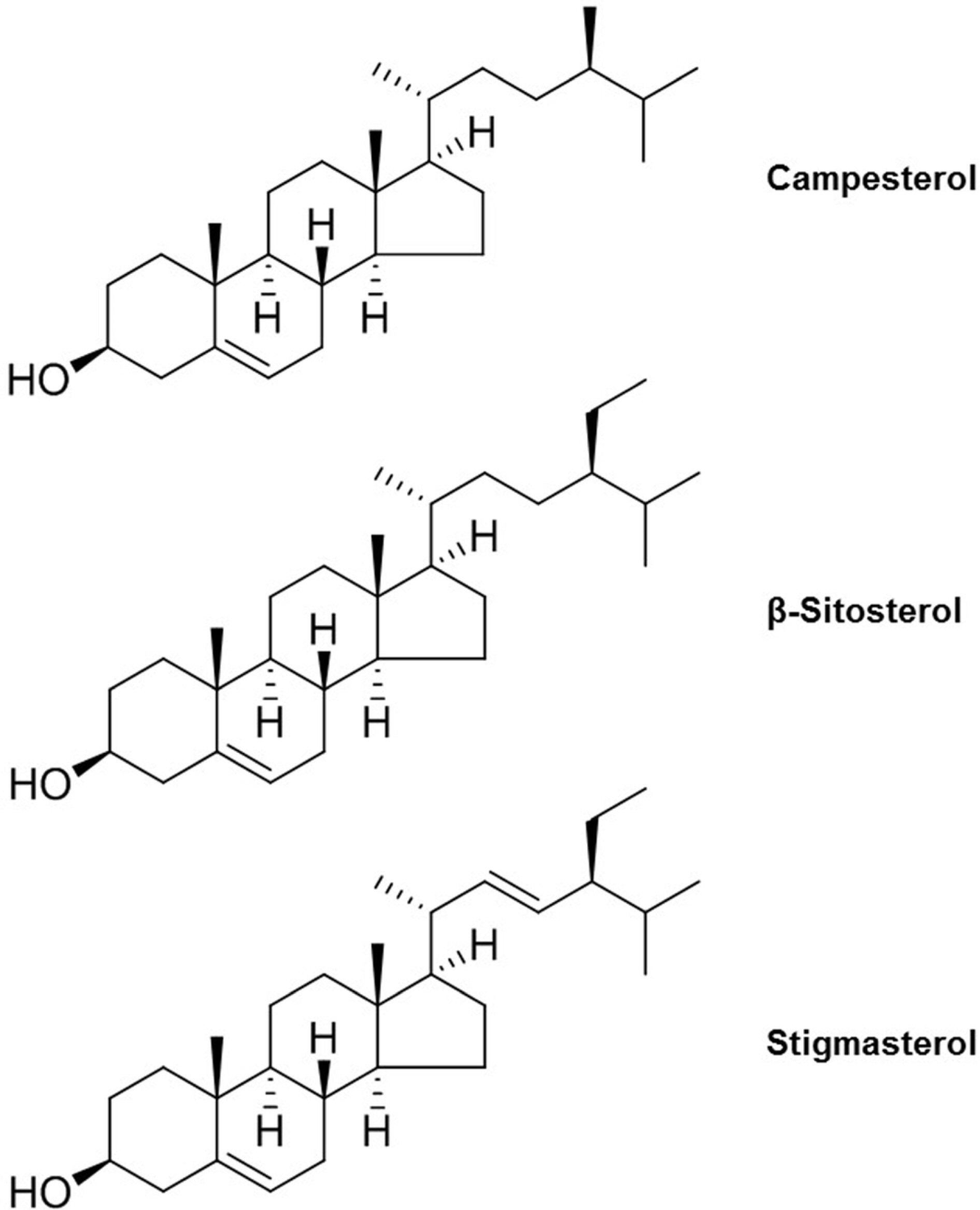
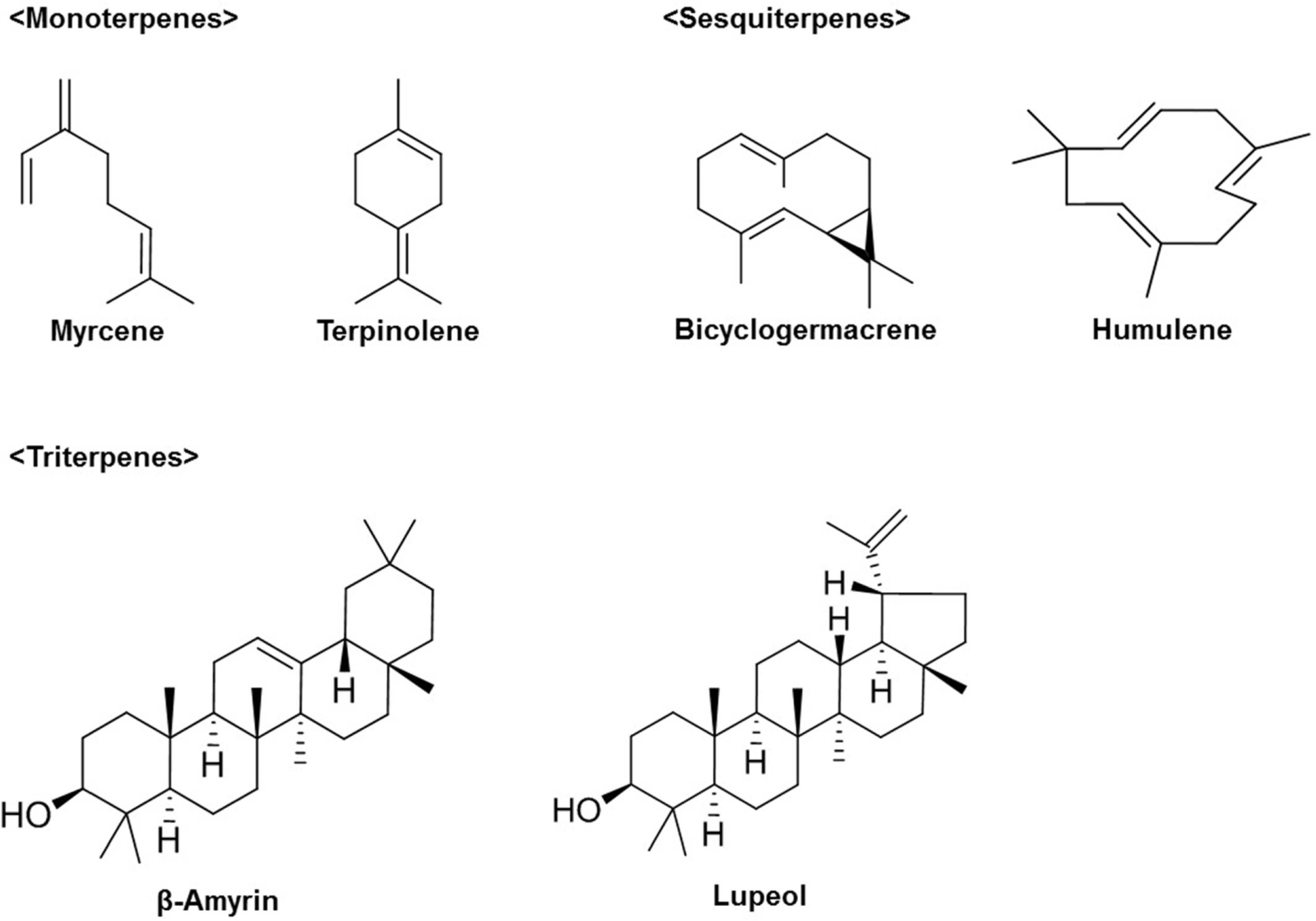
- Ginseng (Panax ginseng) is recognized as one of the most valuable medicinal herbs in Asia and it contains diverse phytochemicals that contribute to its pharmacological effects. Although lipids represent a major component of ginseng, ginseng lipids are still far from being fully explored. This review is focused on ginseng lipid components and methodologies of their analysis. The ginseng lipid compounds were categorized according to the structural features of each lipid class. This basic information on ginseng lipid components and the analysis methods will be applicable to authentification or quality control of ginseng products, and development of lipid-based pharmaceuticals and nutraceuticals from ginseng.
Figure
Reference
-
References
(1). Cho C. -W.., Kim Y. -C.., Rhee Y. -K.., Lee Y. -C.., Kim K. -T.., Hong H. -D. J.Ethn. Foods. 2014. 1:24–28.(2). Hou J. P.Am. J. Chin. Med. 1977. 5:123–145.(3). Shin B. K.., Kwon S. W.., Park J. H. J.Ginseng Res. 2015. 39:287–298.(4). Qi L. W.., Wang C. Z.., Yuan C. S.Phytochemistry. 2011. 72:689–699.(5). Leung K. W.., Wong A. S.Chin. Med. 2010. 5:20.(6). Lü J. M.., Yao Q.., Chen C.Curr. Vasc. Pharmacol. 2009. 7:293–302.(7). Hwang W. I.., Oh S. K.Korean J. Ginseng Res. 1984. 8:153–166.(8). Kim B.S.., Lee S.Y.., Yun Y.S.., Yun T.K.BMB Rep. 1986. 19:203–217.(9). Kim Y.S.., Kim S.I.., Hahn D.R.Yakhak Hoeji. 1988. 32:137–140.(10). Lee S. H.., Hwang W. I.Korean J. Ginseng Sci. 1986. 10:141–150.(11). Sohn J.., Lee C.H.., Chung D.J.., Park S.H.., Kim I.., Hwang W.I.Exp. Mol. Med. 1998. 30:47–51.(12). Kang M. R.., Kim H. M.., Kang J. S.., Lee K.., Lee S. D.., Hyun D. H.., In M. J.., Park S. K.., Kim D. C.Plant Foods Hum. Nutr. 2011. 66:101–106.(13). Lee S. D.., Park S. K.., Lee E. S.., Kim H. M.., Lee C. W.., Lee K.., Lee K. H.., Kang M. R.., Lee K. S.., Lee J.., Hwang W. I.., Kim D. C. J.Med. Food. 2010. 13:1–5.(14). Lee S. D.., Yoo G.., Chae H. J.., In M. J.., Oh N. S.., Hwang Y. K.., Hwang W. I.., Kim D. C. J.Am. Oil Chem. Soc. 2009. 86:1065–1071.(15). Matsunaga H.., Katano M.., Yamamoto H.., Mori M.., Takata K.Chem. Pharm. Bull. 1989. 37:1279–1281.(16). Matsunaga H.., Katano M.., Yamamoto H.., Fujito H.., Mori M.., Takata K.Chem. Pharm. Bull. 1990. 38:3480–3482.(17). Maeda N.., Kokai Y.., Ohtani S.., Hada T.., Yoshida H.., Mizushina Y.Food Chem. 2009. 112:205–210.(18). Matsubara K.., Matsumoto H.., Mizushina Y.., Mori M.., Nakajima N.., Fuchigami M.., Yoshida H.., Hada T.Oncol. Rep. 2005. 14:157–160.(19). Fahy E.., Subramaniam S.., Brown H. A.., Glass C. K.., Merrill A. H. Jr.., Murphy R. C.., Raetz C. R. H.., Russell D. W.., Seyama Y.., Shaw W.., Shimizu T.., Spener F.., van Meer G.., VanNieuwenhze M. S.., White S. H.., Witztum J. L.., Dennis E. A. J.Lipid Res. 2005. 46:839–862.(20). Fahy E.., Subramaniam S.., Murphy R. C.., Nishijima M.., Raetz C. R.., Shimizu T.., Spener F.., van Meer G.., Wakelam M. J.., Dennis E. A. J.Lipid Res. 2009. 50:S9–S14.(21). Fouillen L.., Colsch B.., Lessire R.Metabolomics coming of age with its technological diversity Vol. 67; Academic Press: London,. 2013. 331–376:. pp.331–376.(22). Cho I. H.., Lee H. J.., Kim Y. S. J.Agric. Food Chem. 2012. 60(7616–7622):185–192.(23). Shin H. S.., Lee M. W.Korean J. Food Sci. Technol. 1980. 12:185–192.(24). Zhang Y.., Lyu X.., Liu T.., Luo J.., Zhang W.., Mu Q.Am. J. Plant Sci. 2013. 4:92–97.(25). Dawid C.., Dunemann F.., Schwab W.., Nothnagel T.., Hofmann T. J.Agric. Food Chem. 2015. 63:9211–9222.(26). Takahashi M.., Yoshikura M.Yakugaku Zasshi. 1964. 84:757–759.
Article(27). Poplawski J.., Wrobel J. T.., Glinka T.Phytochemistry. 1980. 19:1539–1541.(28). Shim S. C.., Koh H. Y.., Han B. H.Phytochemistry. 1983. 22:1817–1818.(29). Hirakura K.., Morita M.., Nakajima K.., Ikeya Y.., Mitsuhashi H.Phytochemistry. 1991. 30:3327–3333.(30). Hirakura K.., Morita M.., Nakajima K.., Ikeya Y.., Mitsuhashi H.Phytochemistry. 1991. 30:4053–4055.(31). Hirakura K.., Morita M.., Nakajima K.., Ikeya Y.., Mitsuhashi H.Phytochemistry. 1992. 31:899–903.(32). Tasaka Y.., Gombos Z.., Nishiyama Y.., Mohanty P.., Ohba T.., Ohki K.., Murata N.EMBO. J. 1996. 15:6416–6425.(33). Bligh E. G.., Dyer W. J.Can. J. Biochem. Physiol. 1959. 37:911–917.(34). Hansen J.., M⊘ller I.Anal. Biochem. 1975. 68:87–94.(35). Kim S. H.., Shin Y. S.., Choi H. K.Anal. Bioanal. Chem. 2016. 408:2109–2121.(36). Matyash V.., Liebisch G.., Kurzchalia T. V.., Shevchenko A.., Schwudke D. J.Lipid Res. 2008. 49:1137–1146.(37). Han X.., Gross R. W.Mass Spectrom. Rev. 2005. 24:367–412.
Article(38). Yemm E. W.., Cocking E. C.., Ricketts R. E.Analyst. 1955. 80:209–214.(39). Bregoff H. M.., Roberts E.., Delwiche C. C. J.Biol. Chem. 1953. 205:565–574.(40). Dittmer J. C.., Lester R. L. J.Lipid Res. 1964. 5:126–127.(41). Dufourc E. J.Plant Signal. Behav. 2008. 3:133–134.(42). Bradford P. G.., Awad A. B.Mol. Nutr. Food Res. 2007. 51:161–170.(43). Jones P. J.., MacDougall D. E.., Ntanios F.., Vanstone C. A.Can. J. Physiol. Pharmacol. 1997. 75:217–227.(44). Matsumoto T.., Akihisa T.., Soma S.., Takido M.., Takahashi S.., Yamanouchi S. J.Am. Oil Chem. Soc. 1986. 63:544–546.(45). Lee M. -H.., Jeong J. -H.., Seo J. -W.., Shin C. -G.., Kim Y. -S.., In J. -G.., Yang D. -C.., Yi J. -S.., Choi Y.-E. Plant Cell Physiol. 2004. 45:976–984.(46). Reineccius G.Flavor Chemistry and Technology (2eds.). CRC press: Boca Raton;2005. p. 123–130.(47). Yu D.., Xu F.., Zeng J.., Zhan J.IUBMB Life. 2012. 64:285–295.
Article(48). Chung I. -M.., Lim J. -J.., Ahn M. -S.., Jeong H. -N.., An T. -J.., Kim S. -H. J.Ginseng Res. 2016. 40:68–75.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Brief Introduction of Panax ginseng C.A. Meyer
- Effect of Ginseng Extract on Blood Lipids and Atherosclerosis
- The Comparison of Seasonal Ginsenoside Composition Contents in Korean Wild Simulated Ginseng (Panax ginseng) which were Cultivated in Different Areas and Various Ages
- The Perception of Ginseng in England and America, 1600-1800
- Influence of Ginseng Powder and Ginseng Saponin on the Growth of Candida albicans in vitro