Korean J Radiol.
2009 Dec;10(6):552-558. 10.3348/kjr.2009.10.6.552.
Gray Matter Concentration Abnormality in Brains of Narcolepsy Patients
- Affiliations
-
- 1Department of Neurology, Sleep Center, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea. sbhong@skku.edu
- 2Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea.
- 3Neuroscience Research Institute, Kangwon National University College of Medicine, Kangwon-do 220-093, Korea.
- KMID: 1102557
- DOI: http://doi.org/10.3348/kjr.2009.10.6.552
Abstract
OBJECTIVE
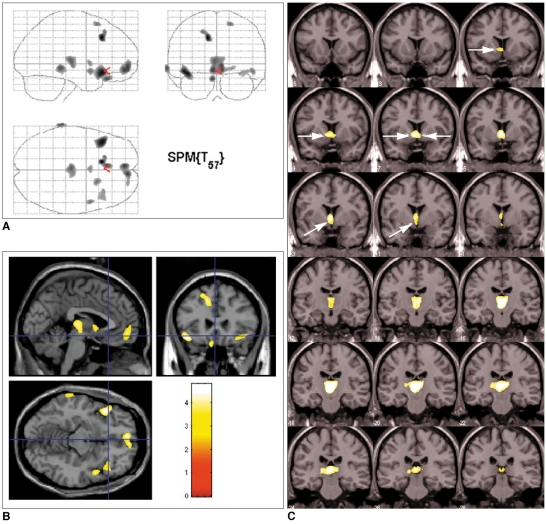
To investigate gray matter concentration changes in the brains of narcoleptic patients. MATERIALS AND METHODS: Twenty-nine narcoleptic patient with cataplexy and 29 age and sex-matched normal subjects (mean age, 31 years old) underwent volumetric MRIs. The MRIs were spatially normalized to a standard T1 template and subdivided into gray matter, white matter, and cerebrospinal fluid (CSF). These segmented images were then smoothed using a 12-mm full width at half maximum (FWHM) isotropic Gaussian kernel. An optimized voxel-based morphometry protocol was used to analyze brain tissue concentrations using SPM2 (statistical parametric mapping). A one-way analysis of variance was applied to the concentration analysis of gray matter images. RESULTS: Narcoleptics with cataplexy showed reduced gray matter concentration in bilateral thalami, left gyrus rectus, bilateral frontopolar gyri, bilateral short insular gyri, bilateral superior frontal gyri, and right superior temporal and left inferior temporal gyri compared to normal subjects (uncorrected p < 0.001). Furthermore, small volume correction revealed gray matter concentration reduction in bilateral nuclei accumbens, hypothalami, and thalami (false discovery rate corrected p < 0.05). CONCLUSION: Gray matter concentration reductions were observed in brain regions related to excessive daytime sleepiness, cognition, attention, and memory in narcoleptics with cataplexy.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Is High IQ Protective Against Cognitive Dysfunction in Narcoleptic Patients?
So-Mee Yoon, Eun Yeon Joo, Ji Young Kim, Kyoung Jin Hwang, Seung Bong Hong
J Clin Neurol. 2013;9(2):118-124. doi: 10.3988/jcn.2013.9.2.118.
Reference
-
1. Guilleminault C, Dement WC. 235 cases of excessive daytime sleepiness. Diagnosis and tentative classification. J Neurol Sci. 1977; 31:13–27. PMID: 188992.2. Plazzi G, Montagna P, Provini F, Bizzi A, Cohen M, Lugaresi E. Pontine lesions in idiopathic narcolepsy. Neurology. 1996; 46:1250–1254. PMID: 8628461.
Article3. Bassetti C, Aldrich MS, Quint DJ. MRI findings in narcolepsy. Sleep. 1997; 20:630–631. PMID: 9351130.
Article4. Draganski B, Geisler P, Hajak G, Schuierer G, Bogdahn U, Winkler J, et al. Hypothalamic gray matter changes in narcoleptic patients. Nat Med. 2002; 8:1186–1188. PMID: 12411926.
Article5. Overeem S, Steens SC, Good CD, Ferrari MD, Mignot E, Frackowiak RS, et al. Voxel-based morphometry in hypocretin-deficient narcolepsy. Sleep. 2003; 26:44–46. PMID: 12627731.6. Kaufmann C, Schuld A, Pollmächer T, Auer DP. Reduced cortical gray matter in narcolepsy: preliminary findings with voxel-based morphometry. Neurology. 2002; 58:1852–1855. PMID: 12084891.
Article7. Brenneis C, Brandauer E, Frauscher B, Schocke M, Trieb T, Poewe W, et al. Voxel-based morphometry in narcolepsy. Sleep Med. 2005; 6:531–536. PMID: 15994127.
Article8. Mignot E, Hayduk R, Black J, Grumet FC, Guilleminault C. HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients. Sleep. 1997; 20:1012–1020. PMID: 9456467.9. Doherty DG, Penzotti JE, Koelle DM, Kwok WW, Lybrand TP, Masewicz S, et al. Structural basis of specificity and degeneracy of T cell recognition: pluriallelic restriction of T cell responses to a peptide antigen involves both specific and promiscuous interactions between the T cell receptor, peptide, and HLA-DR. J Immunol. 1998; 161:3527–3535. PMID: 9759873.10. Chabas D, Taheri S, Renier C, Mignot E. The genetics of narcolepsy. Annu Rev Genomics Hum Genet. 2003; 4:459–483. PMID: 14527309.
Article11. Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. An unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapping. 1996; 4:58–73.12. Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000; 27:469–474. PMID: 11055430.
Article13. Ashburner J, Friston KJ. Voxel-based morphometry -- the methods. Neuroimage. 2000; 11:805–821. PMID: 10860804.14. Parent A, Carpenter MB. Human neuroanatomy. 1995. Baltimore, MD: Williams & Wilkins.15. Bourgin P, Huitron-Resendiz S, Spier AD, Fabre V, Morte B, Criado JR, et al. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci. 2000; 20:7760–7765. PMID: 11027239.
Article16. Joo EY, Tae WS, Kim JH, Kim BT, Hong SB. Glucose hypometabolism of hypothalamus and thalamus in narcolepsy. Ann Neurol. 2004; 56:437–440. PMID: 15349874.
Article17. Joo EY, Hong SB, Tae WS, Kim JH, Han SJ, Cho YW, et al. Cerebral perfusion abnormality in narcolepsy with cataplexy. Neuroimage. 2005; 28:410–416. PMID: 16098766.
Article18. Lodi R, Tonon C, Vignatelli L, Iotti S, Montagna P, Barbiroli B, et al. In vivo evidence of neuronal loss in the hypothalamus of narcoleptic patients. Neurology. 2004; 63:1513–1515. PMID: 15505179.
Article19. Groenewegen HJ, Uylings HB. The prefrontal cortex and the integration of sensory, limbic and autonomic information. Prog Brain Res. 2000; 126:3–28. PMID: 11105636.
Article20. Heimer L. A new anatomical framework for neuropsychiatric disorders and drug abuse. Am J Psychiatry. 2003; 160:1726–1739. PMID: 14514480.
Article21. American Academy of Sleep Medicine. The International Classification of Sleep Disorders. Diagnostic & Coding Manual. 2005. 2nd ed. Westchester: The American Academy of Sleep Medicine.22. Percheron G, François C, Talbi B, Meder JF, Fenelon G, Yelnik J. The primate motor thalamus analysed with reference to subcortical afferent territories. Stereotact Funct Neurosurg. 1993; 60:32–41. PMID: 8511432.
Article23. Sadikot AF, Parent A, François C. Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a PHA-L study of subcortical projections. J Comp Neurol. 1992; 315:137–159. PMID: 1372010.
Article24. Akopyan NS, Baklavadzhyan OG, Sarkisyan NV. The effects of the mediodorsal nucleus of the thalamus on respiratory neurons of the medulla oblongata and respiration in rats in conditions of hypoxia. Neurosci Behav Physiol. 2000; 30:449–453. PMID: 10981949.
Article25. Lishman A. Organic Psychiatry. 1998. 3rd ed. Oxford: Blackwell.26. Chikama M, McFarland NR, Amaral DG, Haber SN. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J Neurosci. 1997; 17:9686–9705. PMID: 9391023.
Article27. Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J Comp Neurol. 1987; 262:271–289. PMID: 3624555.
Article28. Bouilleret V, Dupont S, Spelle L, Baulac M, Samson Y, Semah F. Insular cortex involvement in mesiotemporal lobe epilepsy: a positron emission tomography study. Ann Neurol. 2002; 51:202–208. PMID: 11835376.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Updates on structural neuroimaging of narcolepsy with cataplexy
- Comparison of ADC Map with Trace Map in the Normal and Infarct Areas of the Brains of Stro ke Patients
- Selective Gray Matter Infarction in the Basal Ganglia Associated With Transient Ischemic Attack
- Gray, White Matter Concentration Changes and Their Correlation with Heterotopic Neurons in Temporal Lobe Epilepsy
- Diffusion Tensor Imaging of Heterotopia: Changes of Fractional Anisotropy during Radial Migration of Neurons