Korean J Radiol.
2010 Feb;11(1):25-36. 10.3348/kjr.2010.11.1.25.
Gray, White Matter Concentration Changes and Their Correlation with Heterotopic Neurons in Temporal Lobe Epilepsy
- Affiliations
-
- 1Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea. sbhong@skku.edu
- 2Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea.
- 3Neuroscience Research Institute, Kangwon National University School of Medicine Kangwon-do 200-701, Korea.
- KMID: 1787011
- DOI: http://doi.org/10.3348/kjr.2010.11.1.25
Abstract
OBJECTIVE
To identify changes in gray and white matter concentrations (GMC, WMC), and their relation to heterotopic neuron numbers in mesial temporal lobe epilepsy (mTLE).
MATERIALS AND METHODS
The gray matter or white matter concentrations of 16 left and 15 right mTLE patients who achieved an excellent surgical outcome were compared with those of 24 healthy volunteers for the left group and with 23 healthy volunteers for the right group, by optimized voxel-based morphometry using unmodulated and modulated images. A histologic count of heterotopic neurons was obtained in the white matter of the anterior temporal lobe originating from the patients' surgical specimens. In addition, the number of heterotopic neurons were tested to determine if there was a correlation with the GMC or WMC.
RESULTS
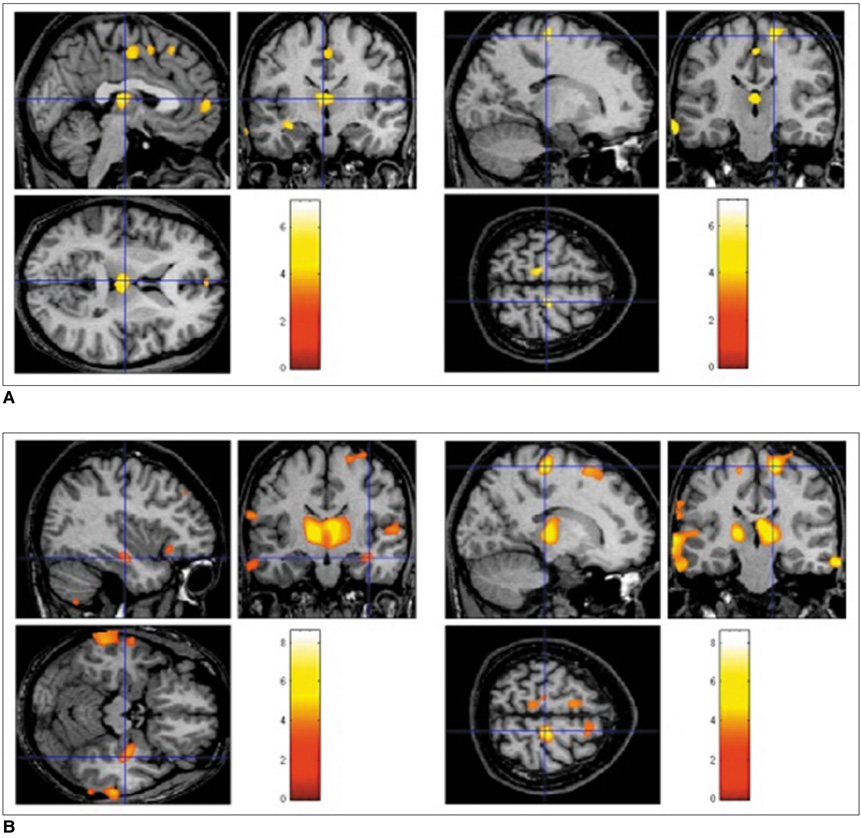
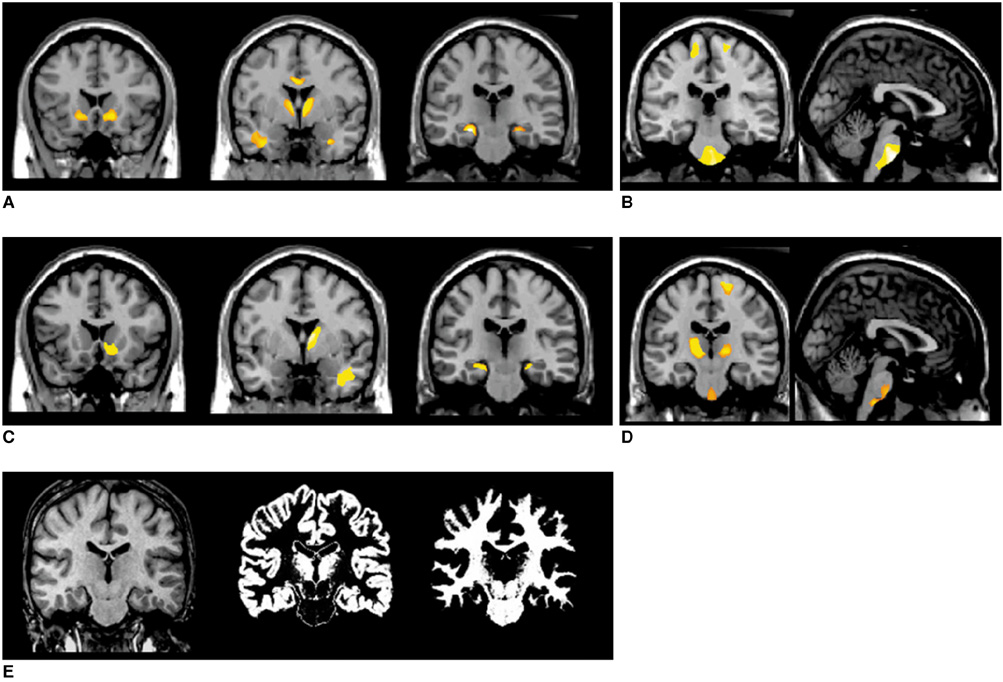
The GMCs of the left and right mTLE groups were reduced in the ipsilateral hippocampi, bilateral thalami, precentral gyri, and in the cerebellum. The WMCs were reduced in the ipsilateral white matter of the anterior temporal lobe, bilateral parahippocampal gyri, and internal capsules, but increased in the pons and bilateral precentral gyri. The heterotopic neuron counts in the left mTLE group showed a positive correlation (r = 0.819, p < 0.0001) with GMCs and a negative correlation (r = -0.839, p < 0.0001) with WMCs in the white matter of the anterior temporal lobe.
CONCLUSION
The present study shows the abnormalities of the cortico-thalamo-hippocampal network including a gray matter volume reduction in the anterior frontal lobes and an abnormality of brain tissue concentration in the pontine area. Furthermore, heterotopic neuron numbers were significantly correlated with GMC or WMC in the left white matter of anterior temporal lobe.
Keyword
MeSH Terms
Figure
Reference
-
1. Bernasconi N, Duchesne S, Janke A, Lerch J, Collins DL, Bernasconi A. Whole-brain voxel-based statistical analysis of gray matter and white matter in temporal lobe epilepsy. Neuroimage. 2004. 23:717–723.2. Bonilha L, Rorden C, Castellano G, Cendes F, Li LM. Voxel-based morphometry of the thalamus in patients with refractory medial temporal lobe epilepsy. Neuroimage. 2005. 25:1016–1021.3. Bonilha L, Rorden C, Castellano G, Pereira F, Rio PA, Cendes F, et al. Voxel-based morphometry reveals gray matter network atrophy in refractory medial temporal lobe epilepsy. Arch Neurol. 2004. 61:1379–1384.4. Wehner T, Luders H. Role of neuroimaging in the presurgical evaluation of epilepsy. J Clin Neurol. 2008. 4:1–16.5. McMillan AB, Hermann BP, Johnson SC, Hansen RR, Seidenberg M, Meyerand ME. Voxel-based morphometry of unilateral temporal lobe epilepsy reveals abnormalities in cerebral white matter. Neuroimage. 2004. 23:167–174.6. Duzel E, Schiltz K, Solbach T, Peschel T, Baldeweg T, Kaufmann J, et al. Hippocampal atrophy in temporal lobe epilepsy is correlated with limbic systems atrophy. J Neurol. 2006. 253:294–300.7. Choi D, Na DG, Byun HS, Suh YL, Kim SE, Ro DW, et al. White-matter change in mesial temporal sclerosis: correlation of MRI with PET, pathology, and clinical features. Epilepsia. 1999. 40:1634–1641.8. Hammers A, Koepp MJ, Hurlemann R, Thom M, Richardson MP, Brooks DJ, et al. Abnormalities of grey and white matter [11C] flumazenil binding in temporal lobe epilepsy with normal MRI. Brain. 2002. 125:2257–2271.9. Tae WS, Joo EY, Kim JH, Han SJ, Suh YL, Kim BT, et al. Cerebral perfusion changes in mesial temporal lobe epilepsy: SPM analysis of ictal and interictal SPECT. Neuroimage. 2005. 24:101–110.10. Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001. 14:21–36.11. Duvernoy HM. The human brain: surface, three-dimensional sectional anatomy, and MRI, and blood supply. 1999. New York: Springer-Verlag.12. Keller SS, Mackay CE, Barrick TR, Wieshmann UC, Howard MA, Roberts N. Voxel-based morphometric comparison of hippocampal and extrahippocampal abnormalities in patients with left and right hippocampal atrophy. Neuroimage. 2002. 16:23–31.13. Keller SS, Wieshmann UC, Mackay CE, Denby CE, Webb J, Roberts N. Voxel based morphometry of grey matter abnormalities in patients with medically intractable temporal lobe epilepsy: effects of side of seizure onset and epilepsy duration. J Neurol Neurosurg Psychiatry. 2002. 73:648–655.14. Mueller SG, Laxer KD, Cashdollar N, Buckley S, Paul C, Weiner MW. Voxel-based optimized morphometry (VBM) of gray and white matter in temporal lobe epilepsy (TLE) with and without mesial temporal sclerosis. Epilepsia. 2006. 47:900–907.15. Keller SS, Cresswell P, Denby C, Wieshmann U, Eldridge P, Baker G, et al. Persistent seizures following left temporal lobe surgery are associated with posterior and bilateral structural and functional brain abnormalities. Epilepsy Res. 2007. 74:131–139.16. Dlugos DJ, Jaggi J, O'Connor WM, Ding XS, Reivich M, O'Connor MJ, et al. Hippocampal cell density and subcortical metabolism in temporal lobe epilepsy. Epilepsia. 1999. 40:408–413.17. Jutila L, Ylinen A, Partanen K, Alafuzoff I, Mervaala E, Partanen J, et al. MR volumetry of the entorhinal, perirhinal, and temporopolar cortices in drug-refractory temporal lobe epilepsy. AJNR Am J Neuroradiol. 2001. 22:1490–1501.18. Lee HW, Hong SB, Tae WS. Opposite ictal perfusion patterns of subtracted SPECT. Hyperperfusion and hypoperfusion. Brain. 2000. 123:2150–2159.19. Rabinowicz AL, Salas E, Beserra F, Leiguarda RC, Vazquez SE. Changes in regional cerebral blood flow beyond the temporal lobe in unilateral temporal lobe epilepsy. Epilepsia. 1997. 38:1011–1014.20. Prince DA, Wilder BJ. Control mechanisms in cortical epileptogenic foci. "Surround" inhibition. Arch Neurol. 1967. 16:194–202.21. Lieb JP, Dasheiff RM, Engel J Jr. Role of the frontal lobes in the propagation of mesial temporal lobe seizures. Epilepsia. 1991. 32:822–837.22. Arfanakis K, Hermann BP, Rogers BP, Carew JD, Seidenberg M, Meyerand ME. Diffusion tensor MRI in temporal lobe epilepsy. Magn Reson Imaging. 2002. 20:511–519.23. Jack CR Jr, Twomey CK, Zinsmeister AR, Sharbrough FW, Petersen RC, Cascino GD. Anterior temporal lobes and hippocampal formations: normative volumetric measurements from MR images in young adults. Radiology. 1998. 172:549–554.24. Jack CR Jr. MRI-based hippocampal volume measurements in epilepsy. Epilepsia. 1994. 35:S21–S29.25. Kim JH, Murdoch GH, Hufnagel TJ, Shen MY, Harrington WN, Spencer DD. White matter changes in intractable temporal lobe epilepsy [abstract]. Epilepsia. 1990. 31:630.26. Chung MH, Horoupian DS. Corpora amylacea: a marker for mesial temporal sclerosis. J Neuropathol Exp Neurol. 1996. 55:403–408.27. Dreifuss S, Vingerhoets FJ, Lazeyras F, Andino SG, Spinelli L, Delavelle J, et al. Volumetric measurements of subcortical nuclei in patients with temporal lobe epilepsy. Neurology. 2001. 57:1636–1641.28. Lee KH, Meador KJ, Park YD, King DW, Murro AM, Pillai JJ, et al. Pathophysiology of altered consciousness during seizures: subtraction SPECT study. Neurology. 2002. 59:841–846.29. Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999. 7:254–266.30. Keller SS, Wilke M, Wieshmann UC, Sluming VA, Roberts N. Comparison of standard and optimized voxel-based morphometry for analysis of brain changes associated with temporal lobe epilepsy. Neuroimage. 2004. 23:860–868.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Increased Corpora Amylacea in the Intractable Temporal Lobe Epilepsy: Case Report
- Diffusion Tensor Imaging of Heterotopia: Changes of Fractional Anisotropy during Radial Migration of Neurons
- Pathologic Analysis of 71 Cases of Cerebral Cortical Dysplasia
- Voxel-based Morphometry (VBM) Based Assessment of Gray Matter Loss in Medial Temporal Lobe Epilepsy: Comparison with FDG PET
- Morphological Alterations of Hippocampus in Temporal Lobe Epilepsy: Cell Loss, Synaptic Reorganization, Cell Birth