Korean J Ophthalmol.
2011 Oct;25(5):355-357. 10.3341/kjo.2011.25.5.355.
Vortex Keratopathy in a Patient Receiving Vandetanib for Non-Small Cell Lung Cancer
- Affiliations
-
- 1Department of Ophthalmology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea. jyhyon@snu.ac.kr
- 2Department of Ophthalmology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1098641
- DOI: http://doi.org/10.3341/kjo.2011.25.5.355
Abstract
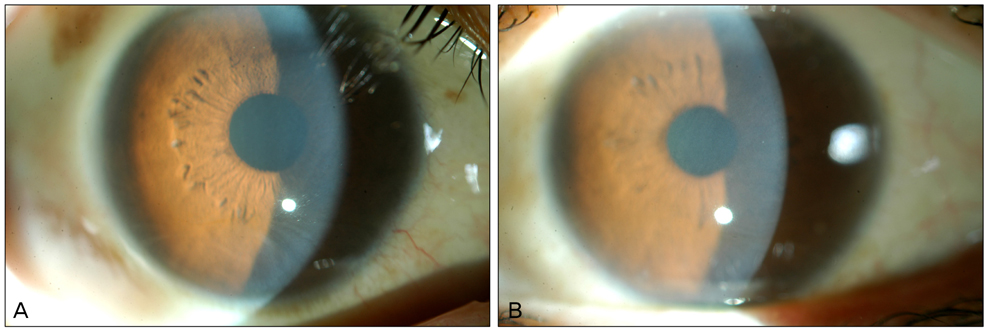
- We report a case of vortex keratopathy in a patient treated with vandetanib for non-small cell lung cancer (NSCLC). A 44-year-old female who underwent two cycles of chemotherapy for NSCLC complained of visual blurring in both eyes after the initiation of vandetanib, an anti-epidermal growth factor receptor (EGFR) and anti-vascular endothelial growth factor receptor 2 protein tyrosine kinase inhibitor. On ophthalmic examination, visual acuities were 20 / 20 OU and, with the exception of diffuse vortex keratopathy in both eyes, other findings were unremarkable. Vandetanib is believed to have caused vortex keratopathy in this patient. Anti-EGFR properties affecting normal corneal epithelial cell migration and wound healing or drug associated metabolite deposition, which is the case in numerous drug-associated vortex keratopathies, may be possible underlying mechanisms in the formation of this corneal complication.
MeSH Terms
-
Adult
Carcinoma, Non-Small-Cell Lung/*drug therapy/pathology
Cornea/drug effects/*pathology
Corneal Diseases/*chemically induced/diagnosis
Diagnosis, Differential
Dose-Response Relationship, Drug
Female
Follow-Up Studies
Humans
Lung Neoplasms/*drug therapy/pathology
Microscopy, Acoustic
Piperidines/administration & dosage/*adverse effects
Quinazolines/administration & dosage/*adverse effects
Visual Acuity
Figure
Cited by 1 articles
-
In Vivo Confocal Microscopic Findings of Corneal Tissue in Amiodarone-Induced Vortex Keratopathy
Jae Jung Lee, Beom Seok Choi, Young Min Park, Jong Soo Lee
J Korean Ophthalmol Soc. 2015;56(1):127-133. doi: 10.3341/jkos.2015.56.1.127.
Reference
-
1. Hollander DA, Aldave AJ. Drug-induced corneal complications. Curr Opin Ophthalmol. 2004. 15:541–548.2. Pallis AG, Serfass L, Dziadziusko R, et al. Targeted therapies in the treatment of advanced/metastatic NSCLC. Eur J Cancer. 2009. 45:2473–2487.3. Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000. 103:211–225.4. Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008. 358:1160–1174.5. Cohen S. The stimulation of epidermal proliferation by a specific protein (EGF). Dev Biol. 1965. 12:394–407.6. Cohen S, Elliott GA. The stimulation of epidermal keratinization by a protein isolated from the submaxillary gland of the mouse. J Invest Dermatol. 1963. 40:1–5.7. Wilson SE, He YG, Weng J, et al. Effect of epidermal growth factor, hepatocyte growth factor, and keratinocyte growth factor, on proliferation, motility and differentiation of human corneal epithelial cells. Exp Eye Res. 1994. 59:665–678.8. Frati L, Daniele S, Delogu A, Covelli I. Selective binding of the epidermal growth factor and its specific effects on the epithelial cells of the cornea. Exp Eye Res. 1972. 14:135–141.9. Savage CR Jr, Cohen S. Proliferation of corneal epithelium induced by epidermal growth factor. Exp Eye Res. 1973. 15:361–366.10. Kasayama S, Ohba Y, Oka T. Expression of the epidermal growth factor gene in mouse lachrymal gland: comparison with that in the submandibular gland and kidney. J Mol Endocrinol. 1990. 4:31–36.11. Kinoshita S. Clinical application of epidermal growth factor in ocular surface disorders. J Dermatol. 1992. 19:680–683.12. Wilson SE, Liang Q, Kim WJ. Lacrimal gland HGF, KGF, and EGF mRNA levels increase after corneal epithelial wounding. Invest Ophthalmol Vis Sci. 1999. 40:2185–2190.13. Nakamura Y, Sotozono C, Kinoshita S. The epidermal growth factor receptor (EGFR): role in corneal wound healing and homeostasis. Exp Eye Res. 2001. 72:511–517.14. Tullo AB, Esmaeli B, Murray PI, et al. Ocular findings in patients with solid tumours treated with the epidermal growth factor receptor tyrosine kinase inhibitor gefitinib ('Iressa', ZD1839) in Phase I and II clinical trials. Eye (Lond). 2005. 19:729–738.15. Yano S, Kondo K, Yamaguchi M, et al. Distribution and function of EGFR in human tissue and the effect of EGFR tyrosine kinase inhibition. Anticancer Res. 2003. 23(5A):3639–3650.16. Zhang G, Basti S, Jampol LM. Acquired trichomegaly and symptomatic external ocular changes in patients receiving epidermal growth factor receptor inhibitors: case reports and a review of literature. Cornea. 2007. 26:858–860.17. Lane K, Goldstein SM. Erlotinib-associated trichomegaly. Ophthal Plast Reconstr Surg. 2007. 23:65–66.18. Yeh S, Fine HA, Smith JA. Corneal verticillata after dual anti-epidermal growth factor receptor and anti-vascular endothelial growth factor receptor 2 therapy (vandetanib) for anaplastic astrocytoma. Cornea. 2009. 28:699–702.19. Stein CA, LaRocca RV, Thomas R, et al. Suramin: an anticancer drug with a unique mechanism of action. J Clin Oncol. 1989. 7:499–508.20. D'Amico DJ, Kenyon KR. Drug-induced lipidoses of the cornea and conjunctiva. Int Ophthalmol. 1981. 4:67–76.21. Lullmann H, Lullmann-Rauch R, Wassermann O. Drug-induced phospholipidoses. II. Tissue distribution of the amphiphilic drug chlorphentermine. CRC Crit Rev Toxicol. 1975. 4:185–218.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stevens-Johnson Syndrome Induced by Vandetanib
- In Vivo Confocal Microscopic Findings of Corneal Tissue in Amiodarone-Induced Vortex Keratopathy
- A Case of Early Gastric Cancer Associated with Small Cell Lung Cancer
- Druggable Targets of Squamous Cell Lung Cancer
- Adjuvant Chemotherapy for Completely Resected Non-Small Cell Lung Cancer