J Korean Ophthalmol Soc.
2015 Jan;56(1):127-133. 10.3341/jkos.2015.56.1.127.
In Vivo Confocal Microscopic Findings of Corneal Tissue in Amiodarone-Induced Vortex Keratopathy
- Affiliations
-
- 1Department of Ophthalmology, Pusan National University School of Medicine, Busan, Korea. jongsool@pusan.ac.kr
- KMID: 2216166
- DOI: http://doi.org/10.3341/jkos.2015.56.1.127
Abstract
- PURPOSE
To analyze the morphology of corneal tissue in patients with Amiodarone-induced vortex keratopathy by in vivo confocal microscopy (IVCM).
CASE SUMMARY
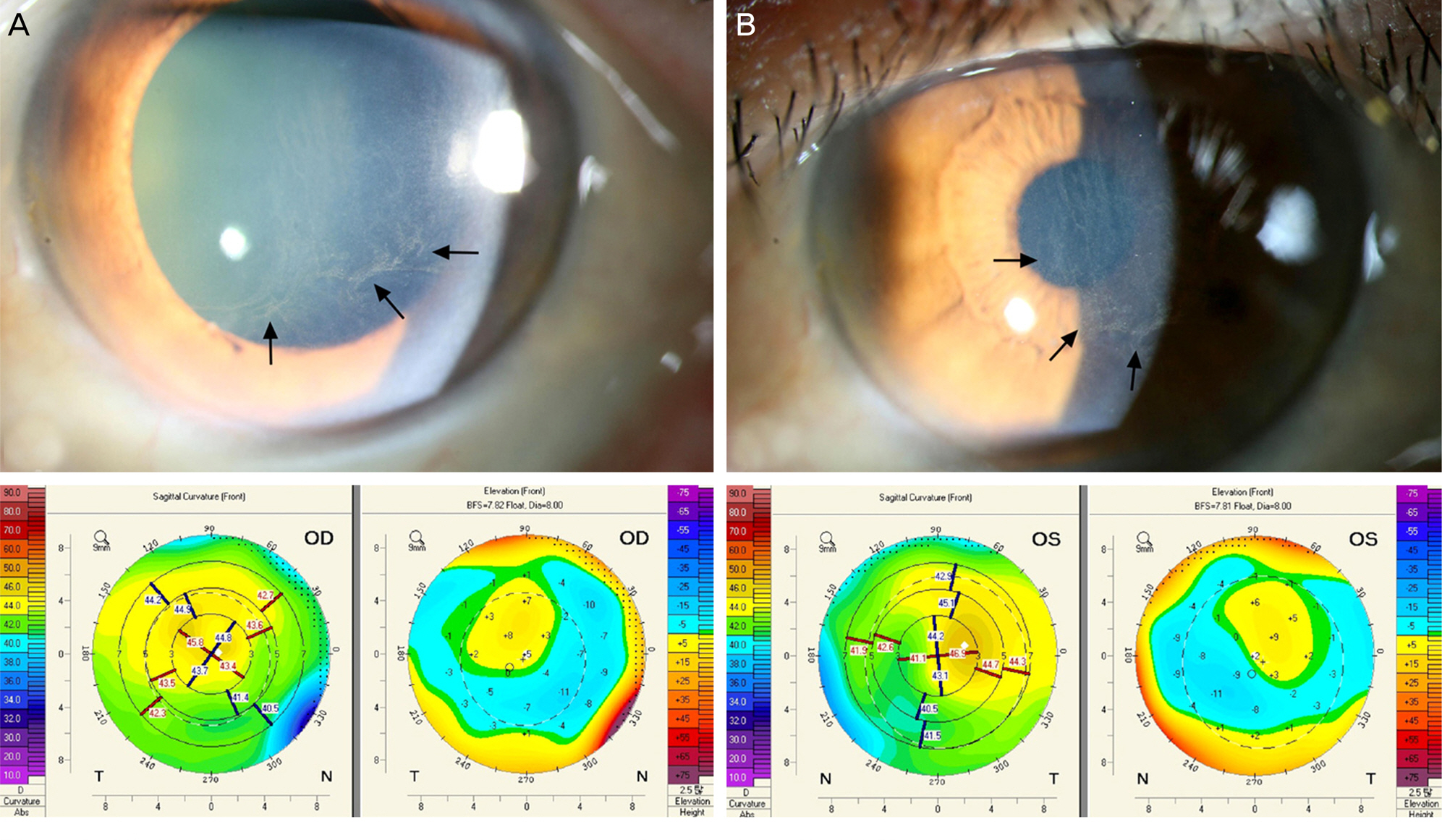
Four eyes of 2 patients with clinically diagnosed Amiodarone-induced vortex keratopathy were examined using corneal topography and IVCM. Cross-sectioned corneal images of the corneal epithelium, Bowman's layer, stromal layer, Descemet's membrane, and endothelium were evaluated. Location of corneal deposits examined by conventional slit-lamp microscopy was correlated with findings of corneal topography. The curvature map of corneal topography revealed an unusual irregular astigmatism with generalized mild steepening consistent with the location of the corneal deposits and the elevation map showed the change of corneal elevation according to the corneal deposits. Multiple hyper-reflective whitish dots were found at the corneal epithelial level and some were found at the anterior stromal level. Regarding the corneal endothelial layer, case 1 demonstrated normal corneal endothelial tissue, but case 2 showed several hyper-reflective whitish dots in the endothelium.
CONCLUSIONS
In patients with Amiodarone-induced vortex keratopathy, IVCM showed corneal deposits in the corneal epithelium, stroma, and endothelium. Distribution of microdeposits in the corneal tissue caused an irregular astigmatism.
MeSH Terms
Figure
Reference
-
References
1. Hollander DA, Aldave AJ. Drug-induced corneal complications. Curr Opin Ophthalmol. 2004; 15:541–8.
Article2. Ahn J, Wee WR, Lee JH, Hyon JY. Vortex keratopathy in a patient receiving vandetanib for non-small cell lung cancer. Korean J Ophthalmol. 2011; 25:355–7.
Article3. Dua HS, Singh A, Gomes JA. . Vortex or whorl formation of cultured human corneal epithelial cells induced by magnetic fields. Eye (Lond). 1996; 10(Pt 4):447–50.
Article4. Ingram DV, Jaggarao NS, Chamberlain DA. Ocular changes resulting from therapy with amiodarone. Br J Ophthalmol. 1982; 66:676–9.
Article5. Mohindra I, Held R, Gwiazda J, Brill J. Astigmatism in infants. Science. 1978; 202:329–31.
Article6. Fulton AB, Dobson V, Salem D. . Cycloplegic refractions in infants and young children. Am J Ophthalmol. 1980; 90:239–47.
Article7. Erdurmus M, Selcoki Y, Yagci R, Hepsen IF. Amiodarone-induced keratopathy: full-thickness corneal involvement. Eye Contact Lens. 2008; 34:131–2.
Article8. Greene HL, Graham EL, Werner JA. . Toxic and therapeutic effects of amiodarone in the treatment of cardiac arrhythmias. J Am Coll Cardiol. 1983; 2:1114–28.
Article9. Wasielica-Poslednik J, Pfeiffer N, Reinke J, Pitz S. Confocal la-ser-scanning microscopy allows differentiation between Fabry disease and amiodarone-induced keratopathy. Graefes Arch Clin Exp Ophthalmol. 2011; 249:1689–96.
Article10. Ciancaglini M, Carpineto P, Zuppardi E. . In vivo confocal mi-croscopy of patients with amiodarone-induced keratopathy. Cornea. 2001; 20:368–73.
Article11. Chakravarti S, Wu F, Vij N. . Microarray studies reveal macro-phage-like function of stromal keratocytes in the cornea. Invest Ophthalmol Vis Sci. 2004; 45:3475–84.
Article12. Dosso A, Rungger-Brändle E. In vivo confocal microscopy in hy-droxychloroquine-induced keratopathy. Graefes Arch Clin Exp Ophthalmol. 2007; 245:318–20.
Article13. Patalano S, Koenig S, Hyndiuk R, Hogatt J. Amiodarone corneal topography. Digit J Ophthalmol. 1997; 3:http://www.djo.harvard.edu/site.php?url=/physicians/oa/391. Accessed November 27, 2014.