J Korean Med Sci.
2005 Apr;20(2):256-261. 10.3346/jkms.2005.20.2.256.
M3 Subtype of Muscarinic Receptors Mediate Ca2+ Release from Intracellular Stores in Rat Prostate Neuroendocrine Cells
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Samsung Medical Center, Seoul, Korea.
- 2Department of Physiology, Sungkyunkwan University School of Medicine, Suwon, Korea.
- 3Department of Physiology and Biophysics, Seoul National University College of Medicine, Seoul, Korea. sjoonkim@snu.ac.kr
- KMID: 1095200
- DOI: http://doi.org/10.3346/jkms.2005.20.2.256
Abstract
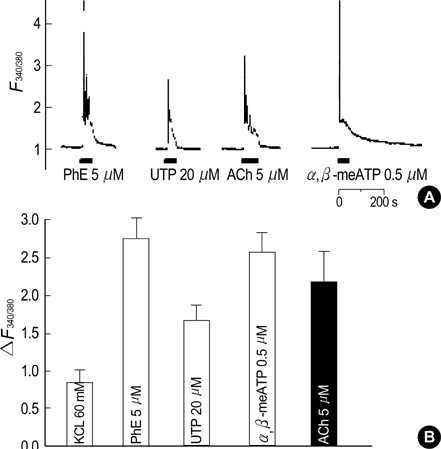
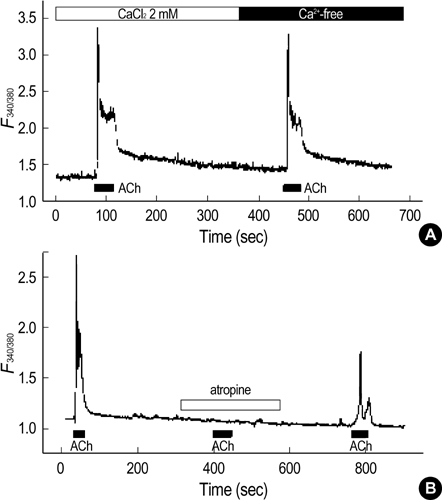
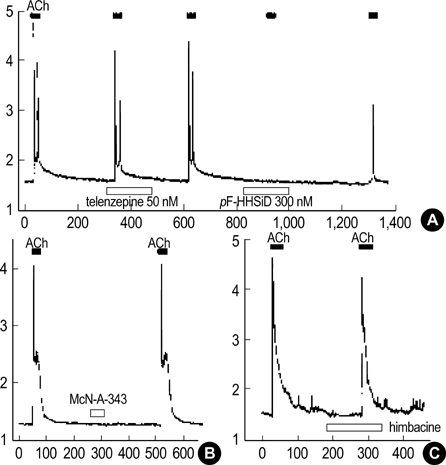
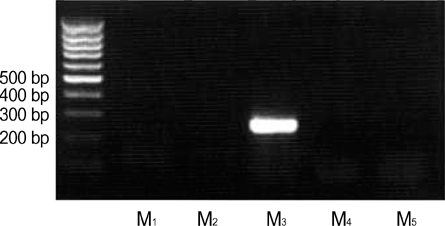
- Our previous studies document the expression of adrenoceptors and purinoceptors in the rat prostate neuroendocrine cells (RPNECs). However, a direct investigation of the receptors for acetylcholine (ACh) is still lacking in the prostate neuroendocrine cells. RPNECs were freshly isolated from the ventral lobes of rat prostate by using collagenase. Effects of ACh and various muscarinic antagonists on the intracellular Ca2+ concentration ([Ca2+]c ) were investigated by using the fura-2 spectrofluorimetry. Single-cell RT-PCR analysis was applied to identify the transcripts for the muscarinic receptor subtypes. ACh (5 micrometer) induced a sharp transient increase in the [Ca2+]c of RPNECs, which was independent of the extracellular Ca2+. In the same RPNECs, high KCl (60 mM), phenylephrine (5micrometer), UTP (P2Y1/2 agonist, 50, micrometer), and alpha, beta-meATP (P2X1/3 agonist, 0.5micrometer) also increased the [Ca2+]c. The ACh-induced [Ca2+]c change (delta[Ca2+]c ) was blocked by atropine or by para-fluorohexahydrosiladifenidol (M3 antagonist, 0.3micrometer), but not by telenzepine (M1 antagonist, 1 micrometer) and himbacine (M2 and M4 antagonist, 1 mircoM). The single-cell RT-PCR demonstrated the selective expression of mRNAs for M3 in RPNECs. In summary, RPNECs express M3 muscarinic receptors that are linked to the release of Ca2+ from intracellular stores. The Ca2+ signals of RPNECs might mediate the parasympathetic regulation of prostate gland.
MeSH Terms
Figure
Reference
-
1. Lee C, Holland JM. Jones TC, Mohr U, Hunt RD, editors. Anatomy, histology, and ultra-structure (correlation with function), Prostate, Rat. Genital System (Monographs on Pathology of Laboratory Animals). 1987. Berlin, Heidelberg: Springer-Verlag;239–251.
Article2. Xue Y, Smedts F, Verhofstad A, Debruyne F, de la Rosette J, Schalken J. Cell kinetics of prostate exocrine and neuroendocrine epithelium and their differential interrelationship: new perspectives. Prostate Suppl. 1998. 8:Suppl. 62–73.
Article3. Cockett AT, di Sant' Agnese PA, Gopinath P, Schoen S, Abrahamsson PA. Relationship of neuroendocrine cells of prostate and serotonin to benign prostatic hyperplasia. Urology. 1993. 42:512–519.
Article4. Abrahamsson PA, Cockett AT, di Sant' Agnese PA. Prognostic significance of neuroendocrine differentiation in clinically localized prostatic carcinoma. Prostate Suppl. 1998. 8:Suppl. 37–42.
Article5. Hong SJ, Kwon SM, Kim SI, Oh HY, Chung BC. Expression of neuroendocrine cells in benign prostate hyperplasia and the effect of dihydrotestosterone. Korean J Urol. 2003. 44:267–271.6. Huggins C, Clark PJ. Quantitative studies of prostatic secretion. II. The effect of castration and of estrogenic injections on the normal and on the hyperplastic prostate glands of dogs. J Exp Med. 1940. 72:747–761.7. McVary KT, McKenna KE, Lee C. Prostate innervation. Prostate Suppl. 1999. 8:Suppl. 2–13.
Article8. Smith ER, Illievski V, Hadidian Z. The stimulation of canine prostatic secretion by pilocarpine. J Pharmacol Exp Ther. 1966. 151:59–65.9. Wang JM, McKenna KE, Lee C. Determination of prostatic secretion in rats: effect of neurotransmitters and testosterone. Prostate. 1991. 18:289–301.
Article10. Smith ER. The stimulation of canine prostatic secretion by sympathomimetic amines. J Pharmacol Exp Ther. 1967. 156:227–231.11. Kim JH, Hong EK, Choi HS, Oh SJ, Kim KM, Uhm DY, Kim SJ. K+ channel currents in rat ventral prostate epithelial cells. Prostate. 2002. 51:201–210.12. Kim JH, Shin SY, Nam JH, Hong EK, Chung YS, Jeong JY, Kang J, Uhm DY, Kim SJ. Adrenergic regulation of the intracellular [Ca2+] and voltage-operated Ca2+ channel currents in the rat prostate neuroendocrine cells. Prostate. 2003. 57:99–110.13. Kim JH, Shin SY, Yun SS, Kim TJ, Oh SJ, Kim KM, Chung YS, Hong EK, Uhm DY, Kim SJ. Voltage-dependent ion channel currents in putative neuroendocrine cells dissociated from the ventral prostate of rat. Pflugers Arch. 2003. 446:88–99.
Article14. Kim SJ, Shin SY, Lee JE, Kim JH, Uhm DY. Ca2+-activated Cl- channel current in rat ventral prostate epithelial cells. Prostate. 2003. 55:118–127.15. Oh SJ, Kim KM, Chung YS, Hong EK, Shin SY, Kim SJ. Ion channel currents of smooth muscle cells isolated from the prostate of guinea-pig. BJU Int. 2003. 92:1022–1030.16. Kim JH, Nam JH, Kim MH, Koh DS, Choi SJ, Kim SJ, Lee JE, Min KM, Uhm DY, Kim SJ. Purinergic receptors coupled to intracellular Ca2+ signals and exocytosis in the rat prostate neuroendocrine cells. J Biol Chem. 2004. 279:27345–27356.17. Polge A, Gaspard C, Mottet N, Guitton C, Boyer JC, Choquet A, Combettes S, Bancel E, Costa P, Bali JP. Neurohormonal stimulation of histamine release from neuroendocrine cells of the human adenomatous prostate. Prostate. 1998. 34:1–9.
Article18. Iismaa TP, Kerr EA, Wilson JR, Carpenter L, Sims N, Biden TJ. Quantitative and functional characterization of muscarinic receptor subtypes in insulin-secreting cell lines and rat pancreatic islets. Diabetes. 2000. 49:392–398.
Article19. Lai J, Shao XM, Pan RW, Dy E, Huang CH, Feldman JL. RT-PCR reveals muscarinic acetylcholine receptor mRNA in the pre-Bötzinger complex. Am J Physiol Lung Cell Mol Physiol. 2001. 281:L1420–L1424.20. Fonteriz RI, Garcia-Sancho J, Gandia L, Lopez MG, Garcia AG. Permeation and inactivation by calcium and manganese of bovine adrenal chromaffin cell calcium channels. Am J Physiol Cell Physiol. 1992. 263:C818–C824.
Article21. Eglen RM, Watson N. Selective muscarinic receptor agonists and antagonists. Pharmacol Toxicol. 1996. 78:59–68.
Article22. Hulme EC, Birdsall NJ, Buckley NJ. Muscarinic receptor subtypes. Ann Rev Pharmacol Toxicol. 1990. 30:633–673.
Article23. Rayford W, Noble MJ, Austenfeld MA, Weigel J, Mebust WK, Shah GV. Muscarinic cholinergic receptors promote growth of human prostate cancer cells. Prostate. 1997. 30:160–166.
Article24. Ventura S, Pennefather J, Mitchelson F. Cholinergic innervation and function in the prostate gland. Pharmacol Ther. 2002. 94:93–112.
Article25. Lepor H, Kuhar MJ. Characterization and localization of the muscarinic cholinergic receptor in human prostate tissue. J Urol. 1984. 132:397–402.26. Ruggieri MR, Colton MD, Wang P, Wang J, Smyth RJ, Pontari MA, Luthin GR. Human prostate muscarinic receptor subtypes. J Pharmacol Exp Ther. 1995. 274:976–982.27. Yazawa H, Honda K. The M3-muscarinic cholinoceptor subtype in rat prostate and its down regulation by aging. Jpn J Pharmacol. 1993. 61:319–324.
Article28. Farrel JI. The newer physiology of the prostate gland. J Urol. 1938. 39:171–185.29. Jacobs SC, Story MT. Exocrine secretion of epidermal growth factor by the rat prostate: effect of adrenergic agents, cholinergic agents, and vasoactive intestinal peptide. Prostate. 1988. 13:79–87.
Article30. Lau WA, Pennefather JN. Muscarinic receptor subtypes in the rat prostate gland. Eur J Pharmacol. 1998. 343:151–156.
Article31. Najbar-Kaszkiel AT, Di Iulio JL, Li CG, Rand MJ. Characterization of excitatory and inhibitory transmitter systems in prostate glands of rats, guinea-pigs, rabbits and pigs. Eur J Pharmacol. 1997. 337:251–258.32. Seki N, Suzuki H. Electrical and mechanical activity of rabbit prostate smooth muscles in response to nerve stimulation. J Physiol (Lond). 1989. 419:651–653.
Article33. Kim JH, Shin SY, Uhm DY, Kim SJ. Effects of noradrenaline on the membrane potential of prostatic neuroendocrine cells of rat. Korean J Physiol Pharmacol. 2003. 7:47–52.34. Pontari MA, Luthin GR, Braverman AS, Ruggieri MR. Characterization of muscarinic cholinergic receptor subtypes in rat prostate. J Recept Signal Transduct Res. 1998. 18:151–166.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ca(2+)-induced Ca2+ Release from Internal Stores in INS-1 Rat Insulinoma Cells
- Effects of Halothane, Enflurane or Isoflurane on the m1 or m3 Muscarinic Receptor Expressed in Xenopus Oocytes
- Muscarinic receptor subtype controlling the carbachol-induced muscle contraction in guinea pig gastric antrum
- The Effect Erythrocyte lysate on Intracellular Calcium Concentration and the Potassium Channel in Cerebral Vasular Smooth Muscle Cells
- Caffeine and 2-Aminoethoxydiphenyl Borate (2-APB) Have Different Ability to Inhibit Intracellular Calcium Mobilization in Pancreatic Acinar Cell