Yonsei Med J.
2010 Sep;51(5):740-745. 10.3349/ymj.2010.51.5.740.
Bone Morphogenetic Protein Receptor in the Osteogenic Differentiation of Rat Bone Marrow Stromal Cells
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P.R. China. anxunwang@yahoo.com
- KMID: 1071427
- DOI: http://doi.org/10.3349/ymj.2010.51.5.740
Abstract
- PURPOSE
Several signaling pathways have been shown to regulate the lineage commitment and terminal differentiation of bone marrow stromal cells (BMSCs). Bone morphogenetic protein (BMP) signaling has important effects on the process of skeletogenesis. In the present study, we tested the role of bone morphogenetic protein receptor (BMPR) in the osteogenic differentiation of rat bone marrow stromal cells in osteogenic medium (OM) with or without BMP-2.
MATERIALS AND METHODS
BMSCs were harvested from rats and cultured in OM containing dexamethasone, beta-glycerophosphate, and ascorbic acid, with or without BMP-2 in order to induce osteogenic differentiation. The alkaline phosphatase (ALP) activity assay and von kossa staining were used to assess the osteogenic differentiation of the BMSCs. BMPR mRNA expression was assessed using reverse transcription-polymerase chain reaction (RT-PCR).
RESULTS
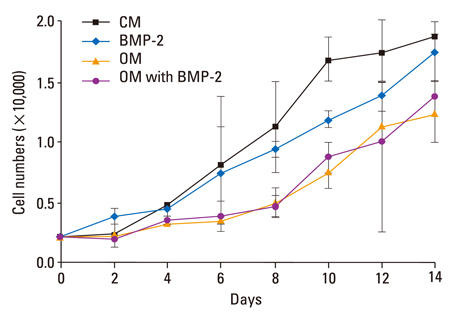
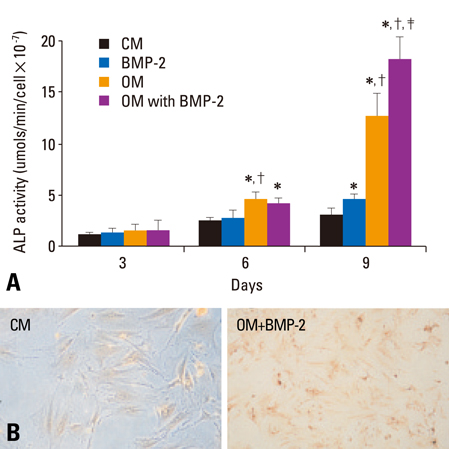
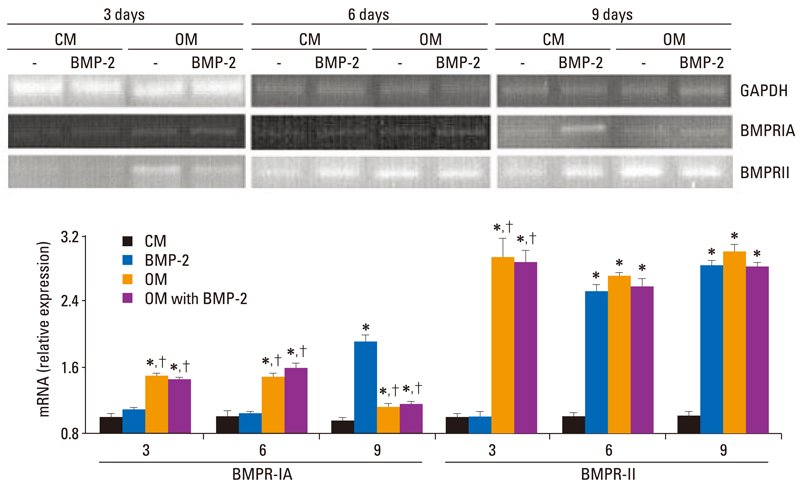
The BMSCs that underwent osteogenic differentiation in OM showed a higher level of ALP activity and matrix mineralization. BMP-2 alone induced a low level of ALP activity and matrix mineralization in BMSCs, but enhanced the osteogenic differentiation of BMSCs when combined with OM. The OM significantly induced the expression of type IA receptor of BMPR (BMPRIA) and type II receptor of BMPR (BMPRII) in BMSCs after three days of stimulation, while BMP-2 significantly induced BMPRIA and BMPRII in BMSCs after nine or six days of stimulation, respectively.
CONCLUSION
BMSCs commit to osteoblastic differentiation in OM, which is enhanced by BMP-2. In addition, BMP signaling through BMPRIA and BMPRII regulates the osteogenic differentiation of rat BMSCs in OM with or without BMP-2.
Keyword
MeSH Terms
-
Alkaline Phosphatase/metabolism
Animals
Bone Marrow Cells/*cytology/drug effects/*metabolism
Bone Morphogenetic Protein 2/pharmacology
Bone Morphogenetic Protein Receptors/genetics/*metabolism
*Cell Differentiation/drug effects
Cell Proliferation/drug effects
Cells, Cultured
Culture Media/pharmacology
Male
Osteogenesis/drug effects/genetics
Rats
Rats, Wistar
Reverse Transcriptase Polymerase Chain Reaction
Stromal Cells/*cytology/drug effects/*metabolism
Figure
Cited by 1 articles
-
Synergistic Effect of Bone Marrow-Derived Mesenchymal Stem Cells and Platelet-Rich Plasma in Streptozotocin-Induced Diabetic Rats
Zhenzhen Lian, Xiaojing Yin, Hua Li, Lili Jia, Xiuzhen He, Yongbo Yan, Naihua Liu, Kayiu Wan, Xiaokun Li, Shaoqiang Lin
Ann Dermatol. 2014;26(1):1-10. doi: 10.5021/ad.2014.26.1.1.
Reference
-
1. Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997. 64:295–312.
Article2. Cheng SL, Zhang SF, Avioli LV. Expression of bone matrix proteins during dexamethasone-induced mineralization of human bone marrow stromal cells. J Cell Biochem. 1996. 61:182–193.
Article3. Heckman JD, Ehler W, Brooks BP, Aufdemorte TB, Lohmann CH, Morgan T, et al. Bone morphogenetic protein but not transforming growth factor-beta enhances bone formation in canine diaphyseal nonunions implanted with a biodegradable composite polymer. J Bone Joint Surg Am. 1999. 81:1717–1729.4. Bi LX, Simmons DJ, Mainous E. Expression of BMP-2 by rat bone marrow stromal cells in culture. Calcif Tissue Int. 1999. 64:63–68.
Article5. Seol YJ, Kim KH, Park YJ, Lee YM, Ku Y, Rhyu IC, et al. Osteogenic effects of bone-morphogenetic-protein-2 plasmid gene transfer. Biotechnol Appl Biochem. 2008. 49:85–96.
Article6. Hsu WK, Sugiyama O, Park SH, Conduah A, Feeley BT, Liu NQ, et al. Lentiviral-mediated BMP-2 gene transfer enhances healing of segmental femoral defects in rats. Bone. 2007. 40:931–938.
Article7. Nishii N, Arai M, Yanai N, Togari A, Nakabayashi T. Effect of bone morphogenetic protein-2 (BMP-2) or troglitazone, as an inducer of osteogenic cells or adipocytes, on differentiation of a bone marrow mesenchymal progenitor cell line established from temperature-sensitive (ts) simian virus (SV) 40 T-antigen gene transgenic mice. Biol Pharm Bull. 2009. 32:10–17.
Article8. Kang Q, Song WX, Luo Q, Tang N, Luo J, Luo X, et al. A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev. 2008. 18:545–559.
Article9. Massagué J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000. 19:1745–1754.10. Ashique AM, Fu K, Richman JM. Signalling via type IA and type IB bone morphogenetic protein receptors (BMPR) regulates intramembranous bone formation, chondrogenesis and feather formation in the chicken embryo. Int J Dev Biol. 2002. 46:243–253.11. Mishina Y, Starbuck MW, Gentile MA, Fukuda T, Kasparcova V, Seedor JG, et al. Bone morphogenetic protein type IA receptor signaling regulates postnatal osteoblast function and bone remodeling. J Biol Chem. 2004. 279:27560–27566.12. Kaps C, Hoffmann A, Zilberman Y, Pelled G, Häupl T, Sittinger M, et al. Distinct roles of BMP receptors Type IA and IB in osteo-/chondrogenic differentiation in mesenchymal progenitors (C3H10T1/2). Biofactors. 2004. 20:71–84.
Article13. Fujii M, Takeda K, Imamura T, Aoki H, Sampath TK, Enomoto S, et al. Roles of bone morphogenetic protein type I receptors and Smad proteins in osteoblast and chondroblast differentiation. Mol Biol Cell. 1999. 10:3801–3813.
Article14. Takeda K, Oida S, Ichijo H, Iimura T, Maruoka Y, Amagasa T, et al. Molecular cloning of rat bone morphogenetic protein (BMP) type IA receptor and its expression during ectopic bone formation induced by BMP. Biochem Biophys Res Commun. 1994. 204:203–209.
Article15. Weston AD, Rosen V, Chandraratna RA, Underhill TM. Regulation of skeletal progenitor differentiation by the BMP and retinoid signaling pathways. J Cell Biol. 2000. 148:679–690.16. Deliloglu-Gurhan SI, Vatansever HS, Ozdal-Kurt F, Tuglu I. Characterization of osteoblasts derived from bone marrow stromal cells in a modified cell culture system. Acta Histochem. 2006. 108:49–57.17. Peter SJ, Liang CR, Kim DJ, Widmer MS, Mikos AG. Osteoblastic phenotype of rat marrow stromal cells cultured in the presence of dexamethasone, beta-glycerolphosphate, and L-ascorbic acid. J Cell Biochem. 1998. 71:55–62.
Article18. Hessle L, Johnson KA, Anderson HC, Narisawa S, Sali A, Goding JW, et al. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci U S A. 2002. 99:9445–9449.
Article19. Anderson HC, Sipe JB, Hessle L, Dhanyamraju R, Atti E, Camacho NP, et al. Impaired calcification around matrix vesicles of growth plate and bone in alkaline phosphatase-deficient mice. Am J Pathol. 2004. 164:841–847.
Article20. Wagner EF, Karsenty G. Genetic control of skeletal development. Curr Opin Genet Dev. 2001. 11:527–532.
Article21. Lee CI, Kohn DB, Ekert JE, Tarantal AF. Morphological analysis and lentiviral transduction of fetal monkey bone marrow-derived mesenchymal stem cells. Mol Ther. 2004. 9:112–123.
Article22. Edgar CM, Chakravarthy V, Barnes G, Kakar S, Gerstenfeld LC, Einhorn TA. Autogenous regulation of a network of bone morphogenetic proteins (BMPs) mediates the osteogenic differentiation in murine marrow stromal cells. Bone. 2007. 40:1389–1398.
Article23. Osyczka AM, Diefenderfer DL, Bhargave G, Leboy PS. Different effects of BMP-2 on marrow stromal cells from human and rat bone. Cells Tissues Organs. 2004. 176:109–119.
Article24. Osyczka AM, Leboy PS. Bone morphogenetic protein regulation of early osteoblast genes in human marrow stromal cells is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase signaling. Endocrinology. 2005. 146:3428–3437.
Article25. Hu Z, Peel SA, Ho SK, Sándor GK, Clokie CM. Role of bovine bone morphogenetic proteins in bone matrix protein and osteoblast-related gene expression during rat bone marrow stromal cell differentiation. J Craniofac Surg. 2005. 16:1006–1014.
Article26. Skillington J, Choy L, Derynck R. Bone morphogenetic protein and retinoic acid signaling cooperate to induce osteoblast differentiation of preadipocytes. J Cell Biol. 2002. 159:135–146.
Article27. Yanagisawa J, Yanagi Y, Masuhiro Y, Suzawa M, Watanabe M, Kashiwagi K, et al. Convergence of transforming growth factor-beta and vitamin D signaling pathways on SMAD transcriptional coactivators. Science. 1999. 283:1317–1321.
Article28. Helvering LM, Sharp RL, Ou X, Geiser AG. Regulation of the promoters for the human bone morphogenetic protein 2 and 4 genes. Gene. 2000. 256:123–138.
Article29. Nishimura R, Kato Y, Chen D, Harris SE, Mundy GR, Yoneda T. Smad5 and DPC4 are key molecules in mediating BMP-2-induced osteoblastic differentiation of the pluripotent mesenchymal precursor cell line C2C12. J Biol Chem. 1998. 273:1872–1879.
Article30. Wan DC, Shi YY, Nacamuli RP, Quarto N, Lyons KM, Longaker MT. Osteogenic differentiation of mouse adipose-derived adult stromal cells requires retinoic acid and bone morphogenetic protein receptor type IB signaling. Proc Natl Acad Sci U S A. 2006. 103:12335–12340.
Article31. Yeh LC, Unda R, Lee JC. Osteogenic protein-1 differentially regulates the mRNA expression of bone morphogenetic proteins and their receptors in primary cultures of osteoblasts. J Cell Physiol. 2000. 185:87–97.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bone-forming peptide-2 derived from BMP-7 enhances osteoblast differentiation from multipotent bone marrow stromal cells and bone formation
- Exploring upregulated genes during osteogenic differentiation of hMSCs
- TNFalpha Increases the Expression of beta2 Adrenergic Receptors in Osteoblasts
- Microarray Analysis of Gene Expression During Differentiation of Human Mesenchymal Stem Cells Treated with Vitamin E in vitro into Osteoblasts
- Bone Morphogenetic Protein 2-Conjugated Silica Particles Enhanced Early Osteogenic Differentiation of Adipose Stem Cells on the Polycaprolactone Scaffold