Cancer Res Treat.
2025 Apr;57(2):457-477. 10.4143/crt.2024.368.
Synergistic Activation of LEPR and ADRB2 Induced by Leptin Enhances Reactive Oxygen Specie Generation in Triple-Negative Breast Cancer Cells
- Affiliations
-
- 1School of Medicine, Nankai University, Tianjin, China
- 2Tianjin Key Laboratory of Oral and Maxillofacial Function Reconstruction, Hospital of Stomatology, Nankai University, Tianjin, China
- KMID: 2566864
- DOI: http://doi.org/10.4143/crt.2024.368
Abstract
- Purpose
Leptin interacts not only with leptin receptor (LEPR) but also engages with other receptors. While the pro-oncogenic effects of the adrenergic receptor β2 (ADRB2) are well-established, the role of leptin in activating ADRB2 in triple-negative breast cancer (TNBC) remains unclear.
Materials and Methods
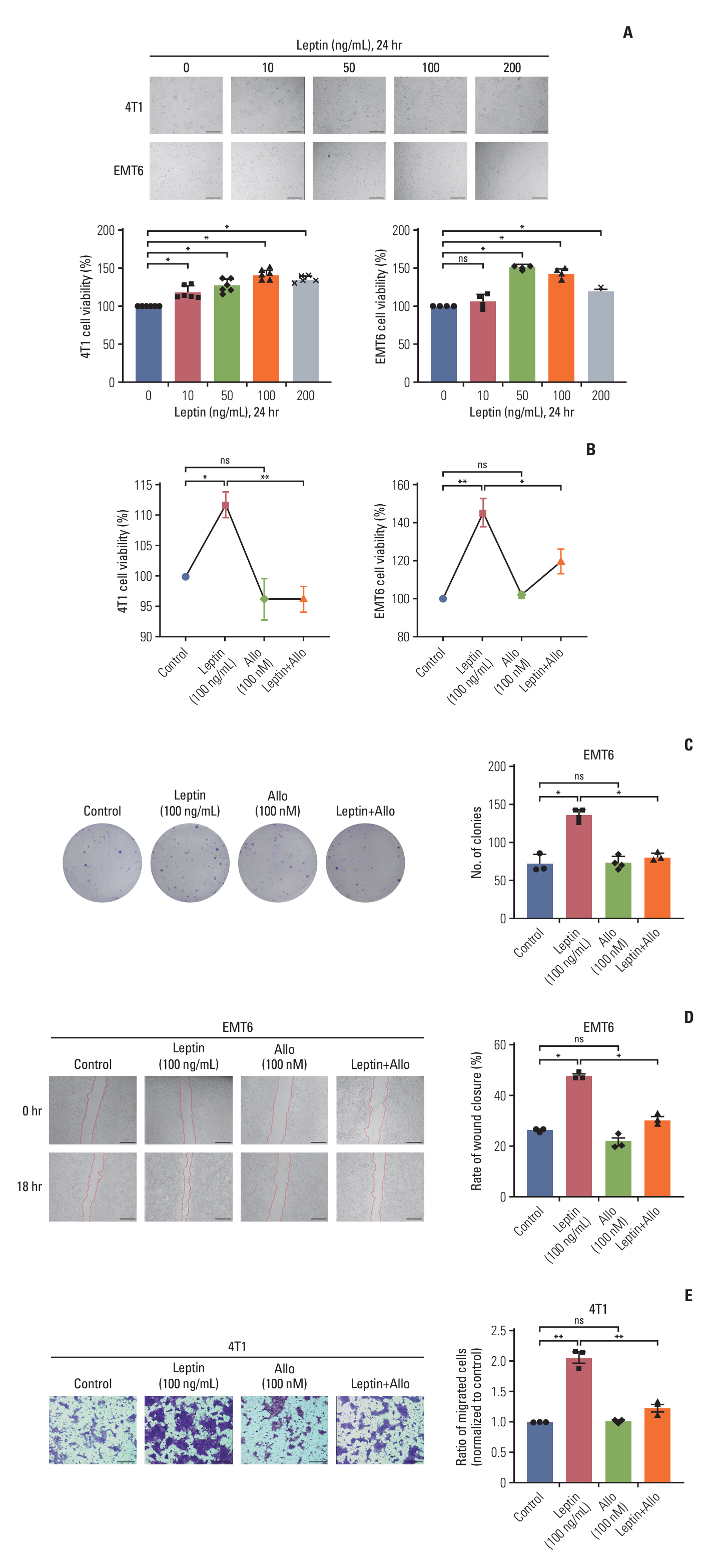
The pro-carcinogenic effects of LEPR were investigated using murine TNBC cell lines, 4T1 and EMT6, and a tumor-bearing mouse model. Expression levels of LEPR, NADPH oxidase 4 (NOX4), and ADRB2 in TNBC cells and tumor tissues were analyzed via western blot and quantitative real-time polymerase chain reaction. Changes in reactive oxygen species (ROS) levels were assessed using flow cytometry and MitoSox staining, while immunofluorescence double-staining confirmed the co-localization of LEPR and ADRB2.
Results
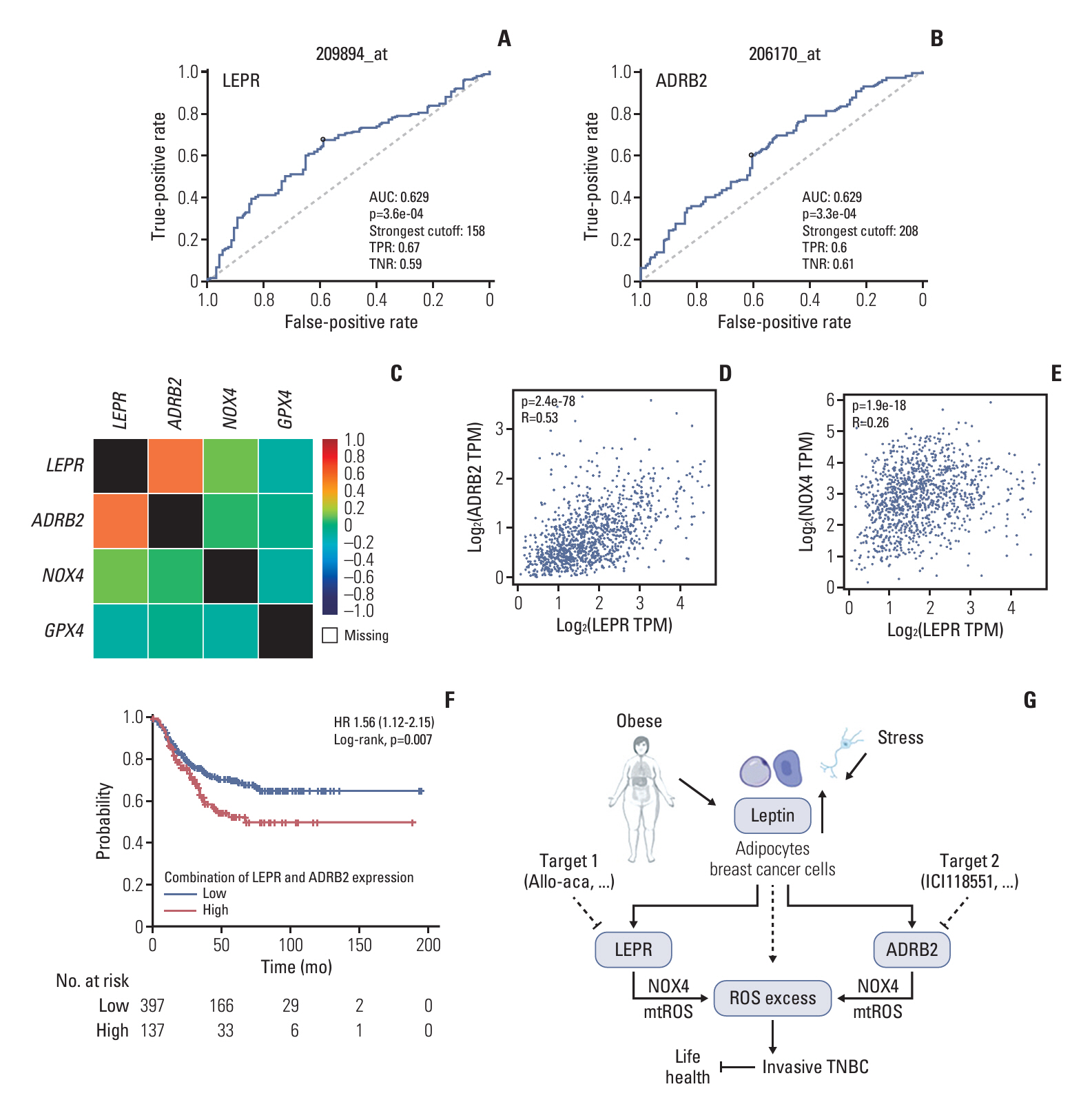
LEPR activation promoted NOX4-derived ROS and mitochondrial ROS production, facilitating TNBC cell proliferation and migration, effects which were mitigated by the LEPR inhibitor Allo-aca. Co-expression of LEPR and ADRB2 was observed on cell membranes, and bioinformatics data revealed a positive correlation between the two receptors. Leptin activated both LEPR and ADRB2, enhancing intracellular ROS generation and promoting tumor progression, which was effectively countered by a specific ADRB2 inhibitor ICI118551. In vivo, leptin injection accelerated tumor growth and lung metastases without affecting appetite, while treatments with Allo-aca or ICI118551 mitigated these effects.
Conclusion
This study demonstrates that leptin stimulates the growth and metastasis of TNBC through the activation of both LEPR and ADRB2, resulting in increased ROS production. These findings highlight LEPR and ADRB2 as potential biomarkers and therapeutic targets in TNBC.
Keyword
Figure
Reference
-
References
1. Lipsyc-Sharf M, Ballman KV, Campbell JD, Muss HB, Perez EA, Shulman LN, et al. Age, body mass index, tumor subtype, and racial and ethnic disparities in breast cancer survival. JAMA Netw Open. 2023; 6:e2339584.
Article2. Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017; 67:378–97.
Article3. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994; 372:425–32.
Article4. Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000; 62:413–37.
Article5. Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995; 83:1263–71.
Article6. Ahima RS, Osei SY. Leptin signaling. Physiol Behav. 2004; 81:223–41.
Article7. Ishikawa M, Kitayama J, Nagawa H. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin Cancer Res. 2004; 10:4325–31.
Article8. Garcia-Estevez L, Calvo I, Perez S, Gallegos I, Diaz E, Sampayo-Cordero M, et al. Predictive role of leptin receptor (ObR) overexpression in patients with early breast cancer receiving neoadjuvant systemic treatment. Cancers (Basel). 2021; 13:3269.
Article9. Sweeney G. Leptin signalling. Cell Signal. 2002; 14:655–63.
Article10. Cleary MP, Phillips FC, Getzin SC, Jacobson TL, Jacobson MK, Christensen TA, et al. Genetically obese MMTV-TGF-alpha/Lep(ob)Lep(ob) female mice do not develop mammary tumors. Breast Cancer Res Treat. 2003; 77:205–15.
Article11. Valentine JM, Ahmadian M, Keinan O, Abu-Odeh M, Zhao P, Zhou X, et al. beta3-Adrenergic receptor downregulation leads to adipocyte catecholamine resistance in obesity. J Clin Invest. 2022; 132:e153357.
Article12. Arner P, Andersson DP, Backdahl J, Dahlman I, Ryden M. Weight gain and impaired glucose metabolism in women are predicted by inefficient subcutaneous fat cell lipolysis. Cell Metab. 2018; 28:45–54.
Article13. Zeng W, Pirzgalska RM, Pereira MM, Kubasova N, Barateiro A, Seixas E, et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell. 2015; 163:84–94.
Article14. Chauveau C, Devedjian JC, Delecourt C, Jeanfils J, Hardouin P, Broux O. Leptin receptors and beta2-adrenergic receptor mRNA expression in brain injury-related heterotopic ossification. J Recept Signal Transduct Res. 2008; 28:347–59.15. Kamiya A, Hayama Y, Kato S, Shimomura A, Shimomura T, Irie K, et al. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat Neurosci. 2019; 22:1289–305.
Article16. Qin JF, Jin FJ, Li N, Guan HT, Lan L, Ni H, et al. Adrenergic receptor beta2 activation by stress promotes breast cancer progression through macrophages M2 polarization in tumor microenvironment. BMB Rep. 2015; 48:295–300.
Article17. Chang A, Botteri E, Gillis RD, Lofling L, Le CP, Ziegler AI, et al. Beta-blockade enhances anthracycline control of metastasis in triple-negative breast cancer. Sci Transl Med. 2023; 15:eadf1147.
Article18. Mahbouli S, Der Vartanian A, Ortega S, Rouge S, Vasson MP, Rossary A. Leptin induces ROS via NOX5 in healthy and neoplastic mammary epithelial cells. Oncol Rep. 2017; 38:3254–64.
Article19. Rios Garcia M, Steinbauer B, Srivastava K, Singhal M, Mattijssen F, Maida A, et al. Acetyl-CoA carboxylase 1-dependent protein acetylation controls breast cancer metastasis and recurrence. Cell Metab. 2017; 26:842–55.
Article20. Saxena NK, Taliaferro-Smith L, Knight BB, Merlin D, Anania FA, O’Regan RM, et al. Bidirectional crosstalk between leptin and insulin-like growth factor-I signaling promotes invasion and migration of breast cancer cells via transactivation of epidermal growth factor receptor. Cancer Res. 2008; 68:9712–22.
Article21. Feola A, Ricci S, Kouidhi S, Rizzo A, Penon A, Formisano P, et al. Multifaceted breast cancer: the molecular connection with obesity. J Cell Physiol. 2017; 232:69–77.
Article22. Giordano C, Vizza D, Panza S, Barone I, Bonofiglio D, Lanzino M, et al. Leptin increases HER2 protein levels through a STAT3-mediated up-regulation of Hsp90 in breast cancer cells. Mol Oncol. 2013; 7:379–91.
Article23. Hu J, Lu R, Zhang Y, Li W, Hu Q, Chen C, et al. beta-adrenergic receptor inhibition enhances oncolytic herpes virus propagation through STAT3 activation in gastric cancer. Cell Biosci. 2021; 11:174.24. Kang Y, Nagaraja AS, Armaiz-Pena GN, Dorniak PL, Hu W, Rupaimoole R, et al. Adrenergic stimulation of DUSP1 impairs chemotherapy response in ovarian cancer. Clin Cancer Res. 2016; 22:1713–24.
Article25. Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, et al. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res. 2006; 12:1447–53.
Article26. Kim HS. Leptin and leptin receptor expression in breast cancer. Cancer Res Treat. 2009; 41:155–63.
Article27. Sanchez-Jimenez F, Perez-Perez A, de la Cruz-Merino L, Sanchez-Margalet V. Obesity and breast cancer: role of leptin. Front Oncol. 2019; 9:596.28. Garcia-Estevez L, Gonzalez-Martinez S, Moreno-Bueno G. The leptin axis and its association with the adaptive immune system in breast cancer. Front Immunol. 2021; 12:784823.29. Ray A. Adipokine leptin in obesity-related pathology of breast cancer. J Biosci. 2012; 37:289–94.
Article30. Munzberg H, Bjornholm M, Bates SH, Myers MG Jr. Leptin receptor action and mechanisms of leptin resistance. Cell Mol Life Sci. 2005; 62:642–52.31. Kimura M, Tateishi N, Shiota T, Yoshie F, Yamauchi H, Suzuki M, et al. Long-term exercise down-regulates leptin receptor mRNA in the arcuate nucleus. Neuroreport. 2004; 15:713–6.
Article32. Yang G, Ge H, Boucher A, Yu X, Li C. Modulation of direct leptin signaling by soluble leptin receptor. Mol Endocrinol. 2004; 18:1354–62.
Article33. Maamra M, Bidlingmaier M, Postel-Vinay MC, Wu Z, Strasburger CJ, Ross RJ. Generation of human soluble leptin receptor by proteolytic cleavage of membrane-anchored receptors. Endocrinology. 2001; 142:4389–93.
Article34. Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol. 2018; 80:50–64.
Article35. Zhao X, Liu M, Li C, Liu X, Zhao J, Ma H, et al. High dose Vitamin C inhibits PD-L1 by ROS-pSTAT3 signal pathway and enhances T cell function in TNBC. Int Immunopharmacol. 2024; 126:111321.
Article36. Zhang Z, Zhang H, Li D, Zhou X, Qin Q, Zhang Q. Caspase-3-mediated GSDME induced pyroptosis in breast cancer cells through the ROS/JNK signalling pathway. J Cell Mol Med. 2021; 25:8159–68.
Article37. Shan M, Qin J, Jin F, Han X, Guan H, Li X, et al. Autophagy suppresses isoprenaline-induced M2 macrophage polarization via the ROS/ERK and mTOR signaling pathway. Free Radic Biol Med. 2017; 110:432–43.
Article38. Yue R, Zhou BO, Shimada IS, Zhao Z, Morrison SJ. Leptin receptor promotes adipogenesis and reduces osteogenesis by regulating mesenchymal stromal cells in adult bone marrow. Cell Stem Cell. 2016; 18:782–96.
Article39. Lu Y, Zhao H, Liu Y, Zuo Y, Xu Q, Liu L, et al. Chronic stress activates plexinA1/VEGFR2-JAK2-STAT3 in vascular endothelial cells to promote angiogenesis. Front Oncol. 2021; 11:709057.
Article40. Rahmouni K, Sigmund CD, Haynes WG, Mark AL. Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes. 2009; 58:536–42.
Article41. Jin M, Wang Y, Zhou T, Li W, Wen Q. Norepinephrine/beta(2)-adrenergic receptor pathway promotes the cell proliferation and nerve growth factor production in triple-negative breast cancer. J Breast Cancer. 2023; 26:268–85.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hydroxyzine Induces Cell Death in Triple-Negative Breast Cancer Cells via Mitochondrial Superoxide and Modulation of Jak2/STAT3 Signaling
- Domperidone Exerts Antitumor Activity in Triple-Negative Breast Cancer Cells by Modulating Reactive Oxygen Species and JAK/STAT3 Signaling
- Effects of Polyamines on TNFalpha- or Tamoxifen-induced Apoptosis in Human Breast Cancer Cells
- Reactive Oxygen Species are Involved in Y-27632-induced Neurite Outgrowth in PC12 Cells
- Role of Calmodulin in the Generation of Reactive Oxygen Species and Apoptosis Induced by Tamoxifen in HepG2 Human Hepatoma Cells