J Korean Neurosurg Soc.
2024 Nov;67(6):609-621. 10.3340/jkns.2024.0053.
Potential Mechanism and Involvement of p120-Catenin in the Malignant Biology of Glioma
- Affiliations
-
- 1Department of Neurosurgery, Tianjin Medical University General Hospital, Tianjin, China
- 2Department of Neurosurgery, Cangzhou Central Hospital, Cangzhou, China
- 3Department of Emergency, Cangzhou Central Hospital, Cangzhou, China
- 4Department of Pathology, Cangzhou Central Hospital, Cangzhou, China
- KMID: 2560403
- DOI: http://doi.org/10.3340/jkns.2024.0053
Abstract
Objective
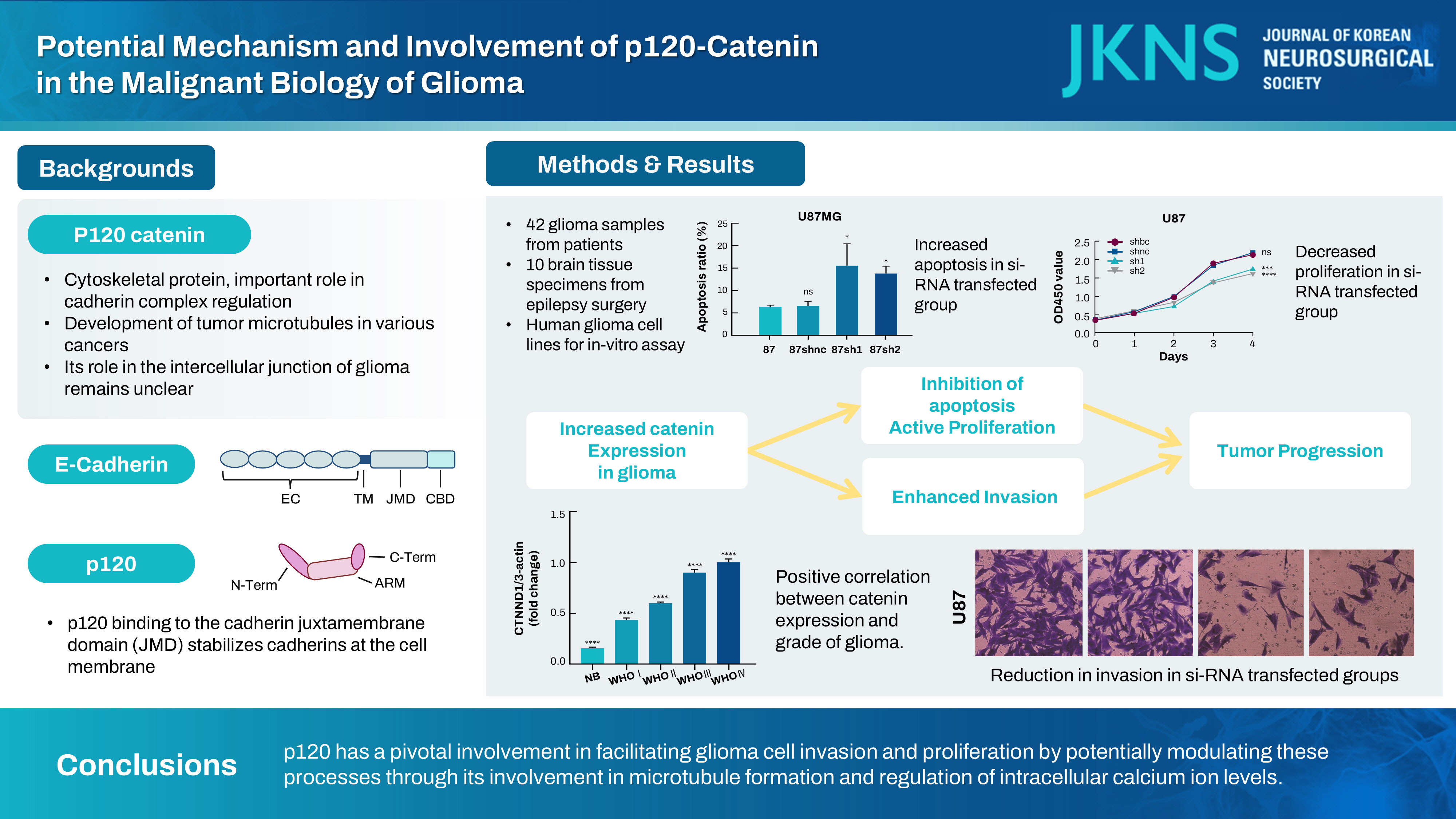
: This study analyzed the influence of p120-catenin (catenin [cadherin-associated protein], delta 1 [CTNND1]) on the malignant characteristics of glioma and elucidated the potential underlying mechanism.
Methods
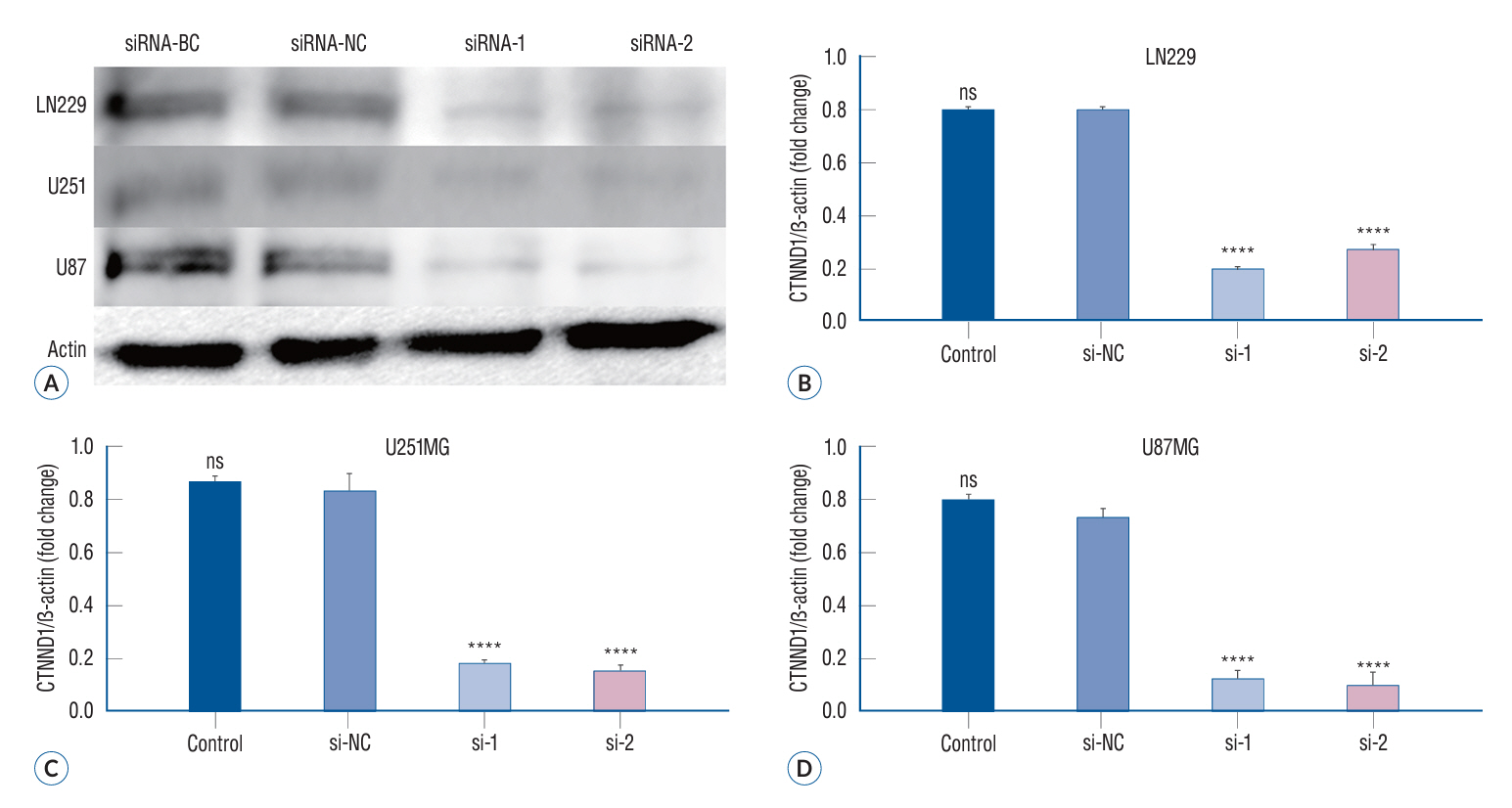
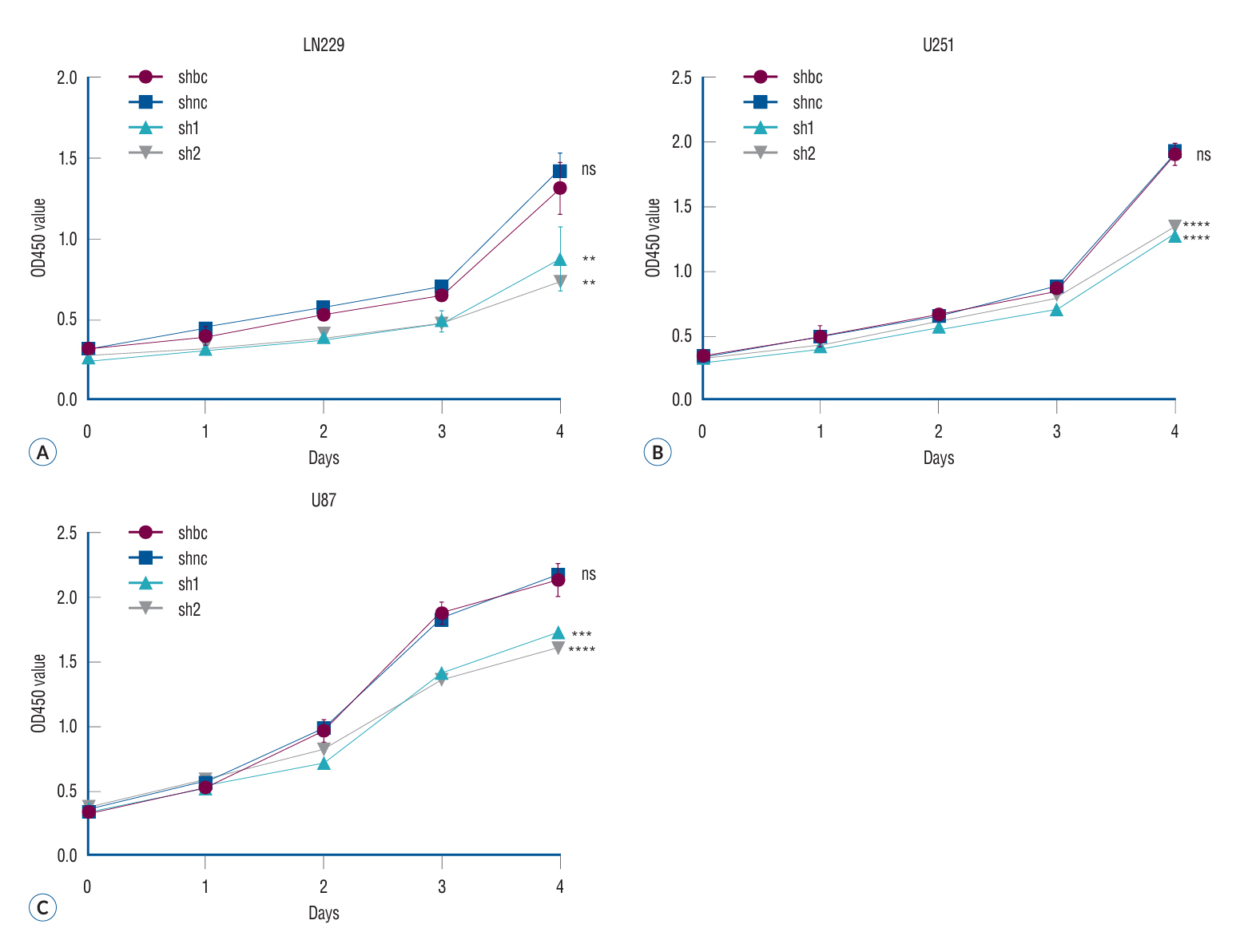
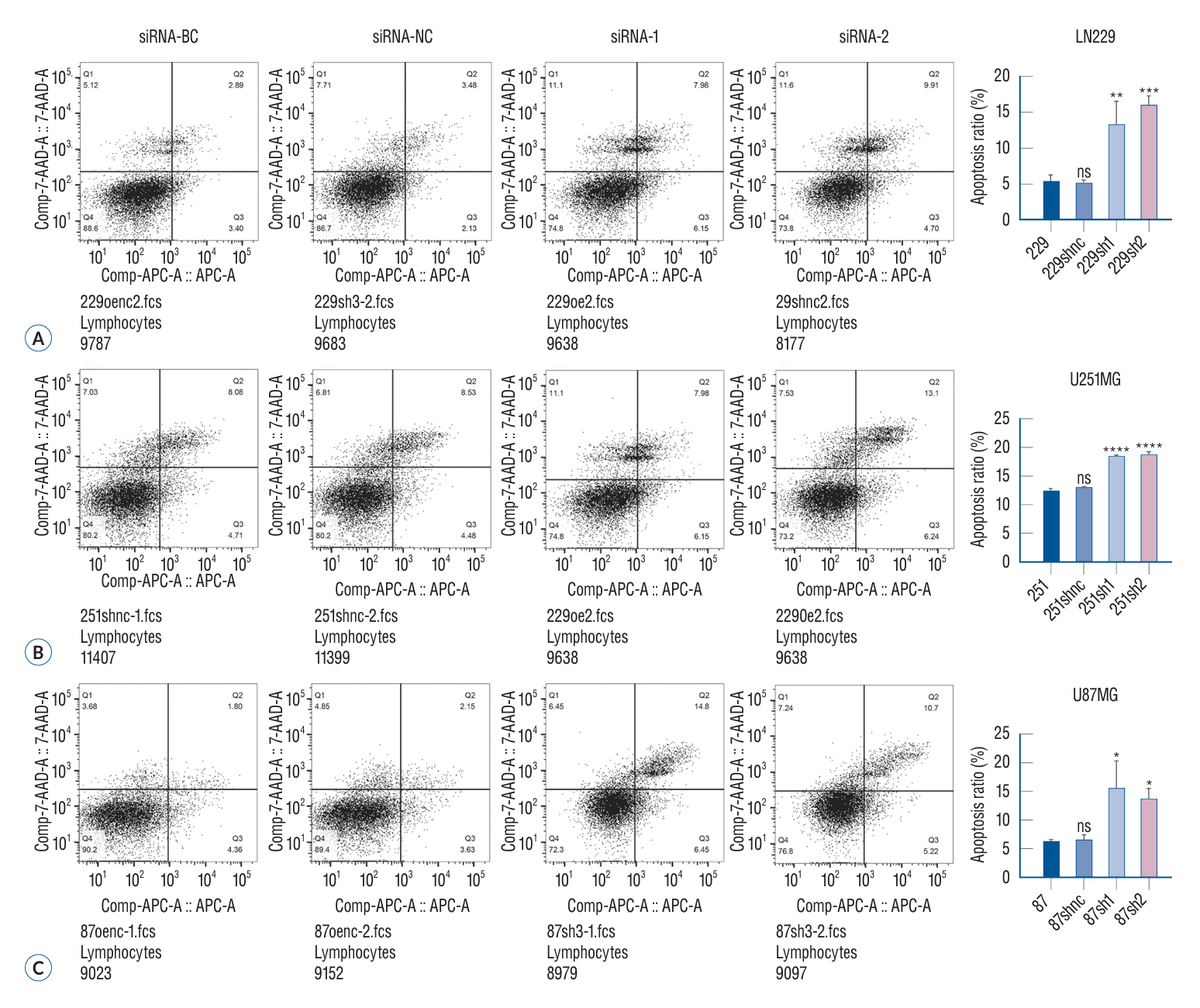
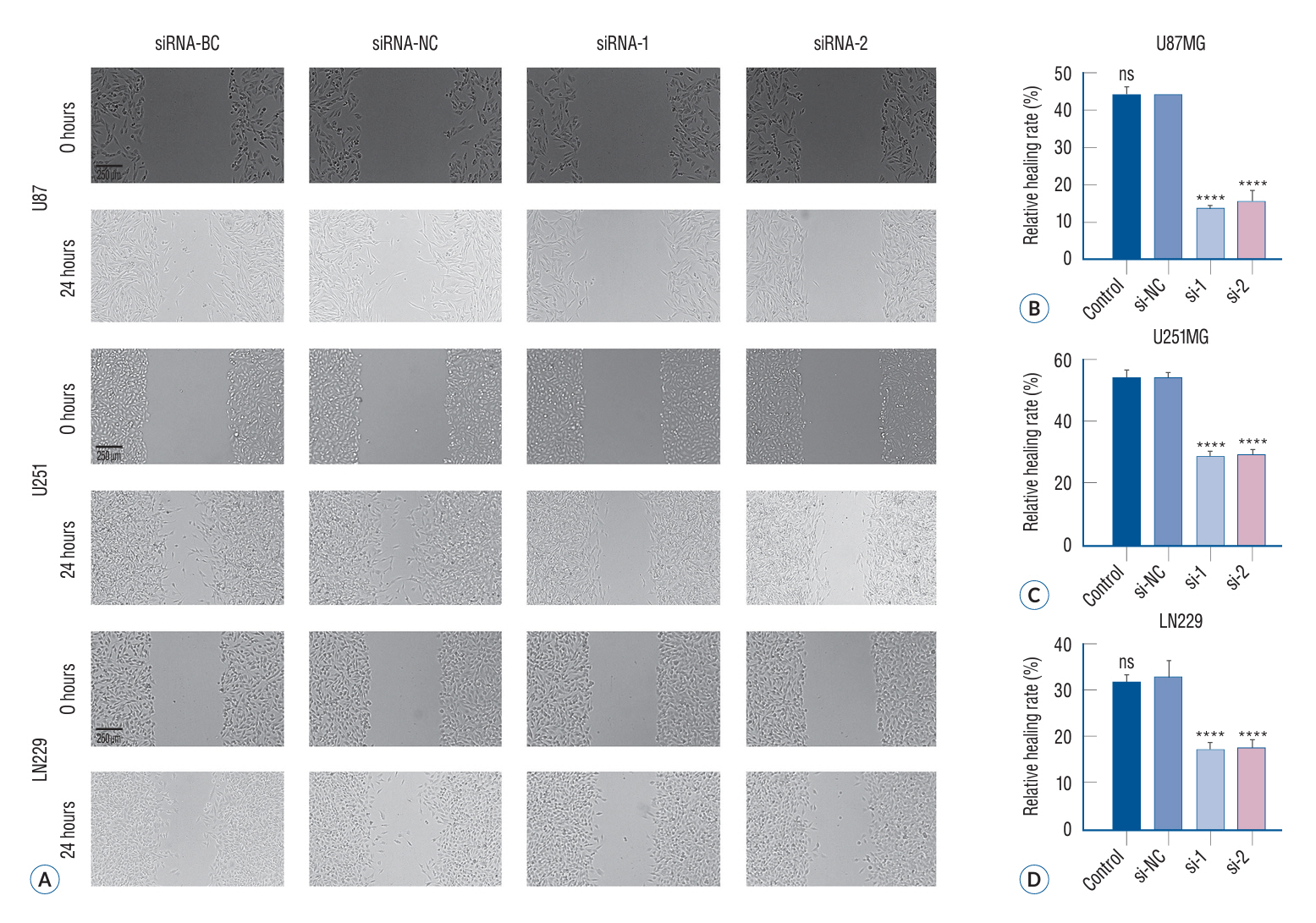
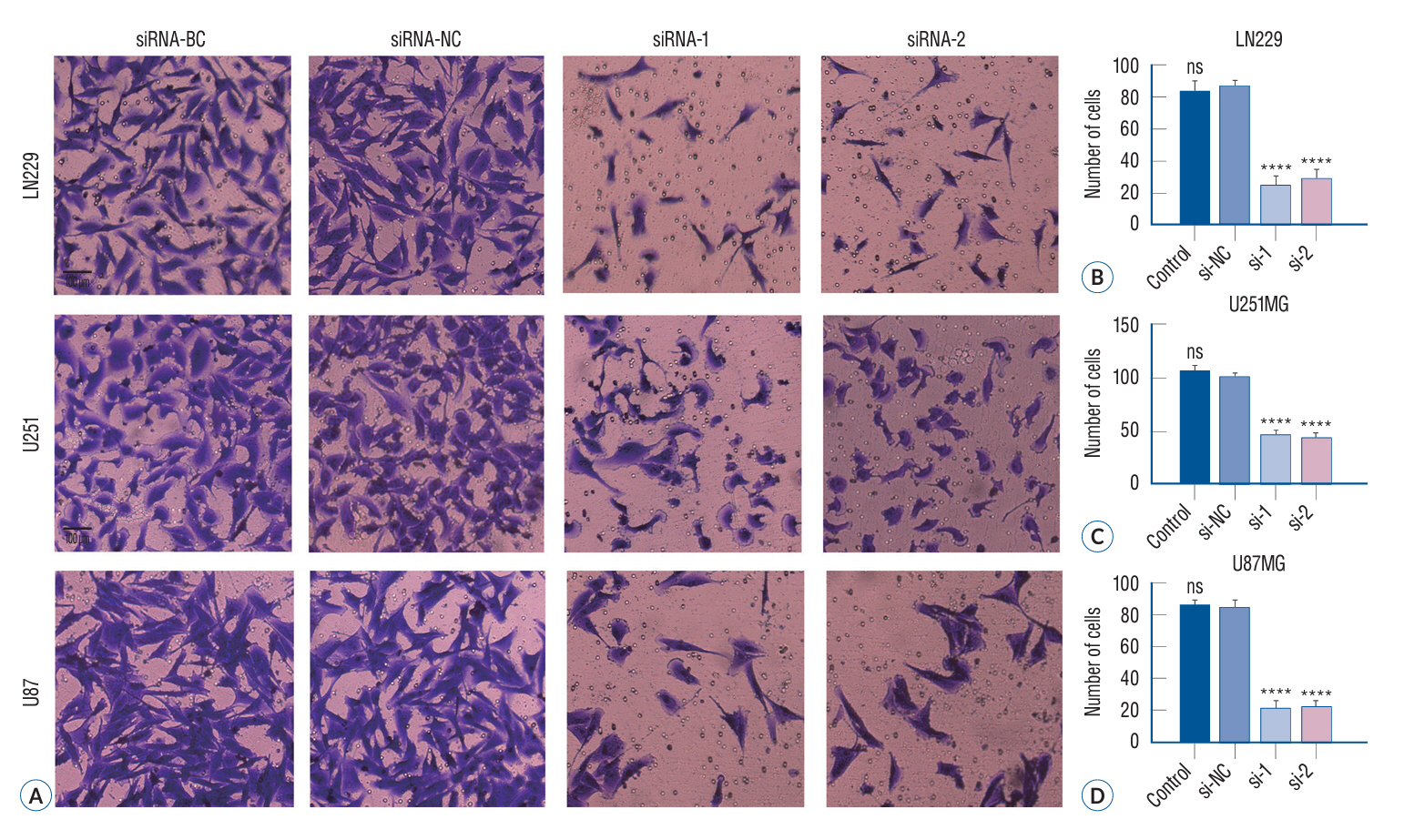
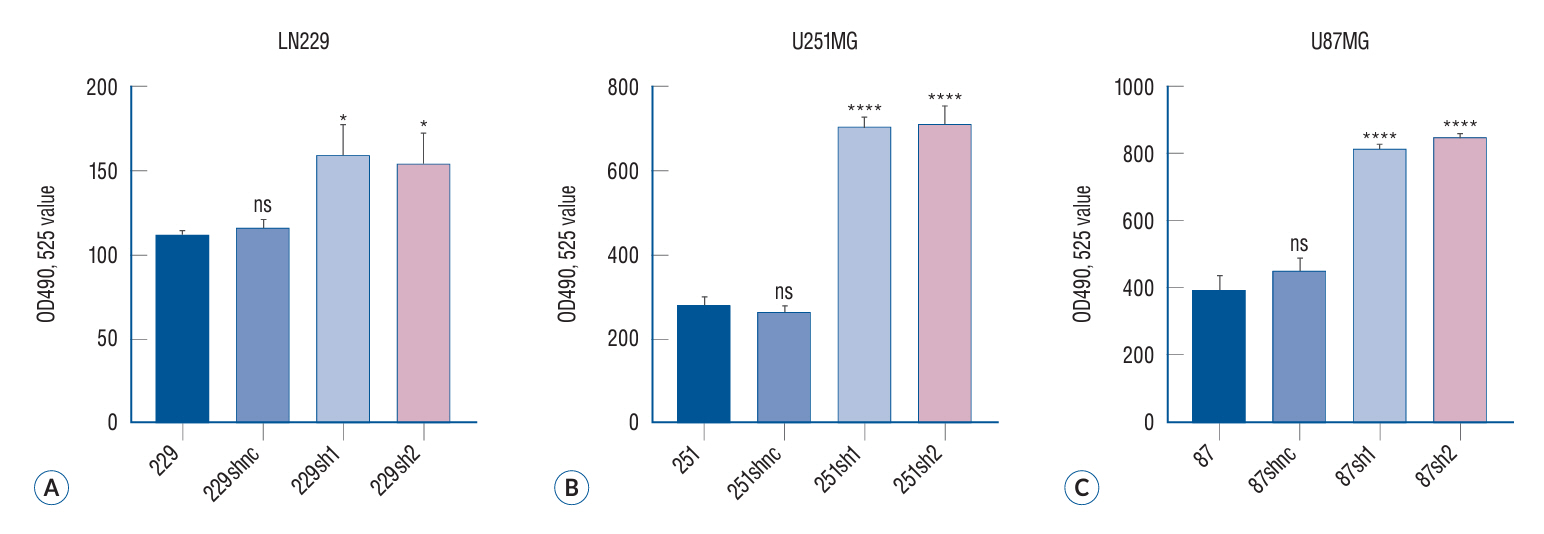
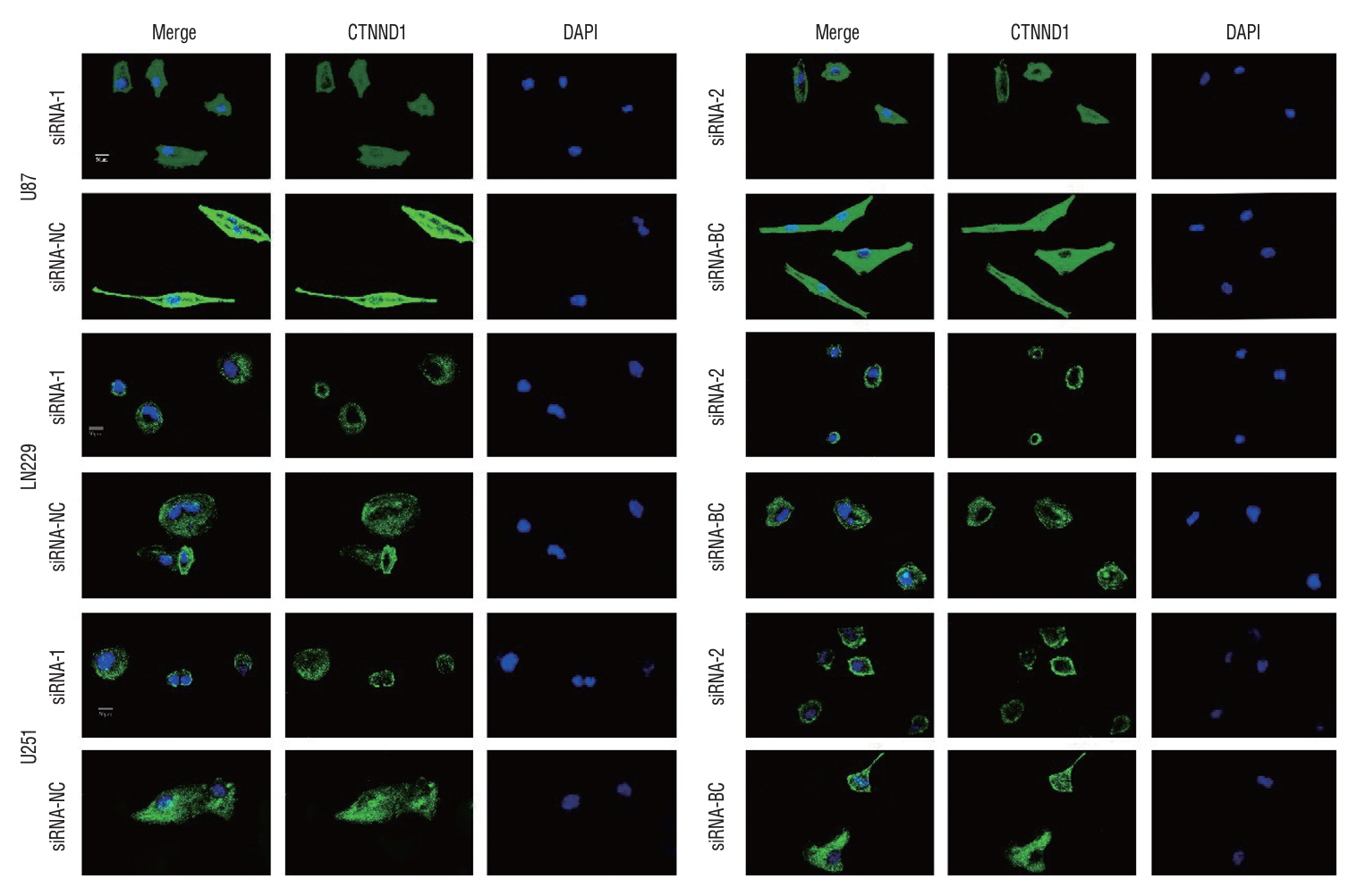
: The p120 expression level was assessed in the brain tissues of 42 glioma patients and 10 patients with epilepsy by using the immunohistochemical method. Meanwhile, quantitative polymerase chain reaction (QT-PCR) technology was employed to assess the expression of p120 in the brain tissues of 71 glioma patients and 13 epilepsy patients. LN229, U251, and U87 glioma cells were used for in vitro analysis and categorized into four treatment groups : siRNA-blank control (BC) group (no RNA sequence was transfected), siRNA-negative control (NC) group (transfected control RNA sequences with no effect), and siRNA-1 and siRNA-2 groups (two p120-specific interfering RNA transfection). p120 expression in these treatment groups was quantified by western blotting assay. The migratory and invasive capabilities of glioma cells were studied by wound healing assay and Transwell invasion assay, respectively, under different treatment conditions. MTT (3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di-phenytetrazoliumromide) assay and cell cycle and apoptosis assay were used to determine glioma cell proliferation and apoptosis, respectively. Enzymelabeled assay was performed to measure intracellular calcium ion concentration. Immunofluorescence assay was performed for determining microtubule formation and glioma cell distribution.
Results
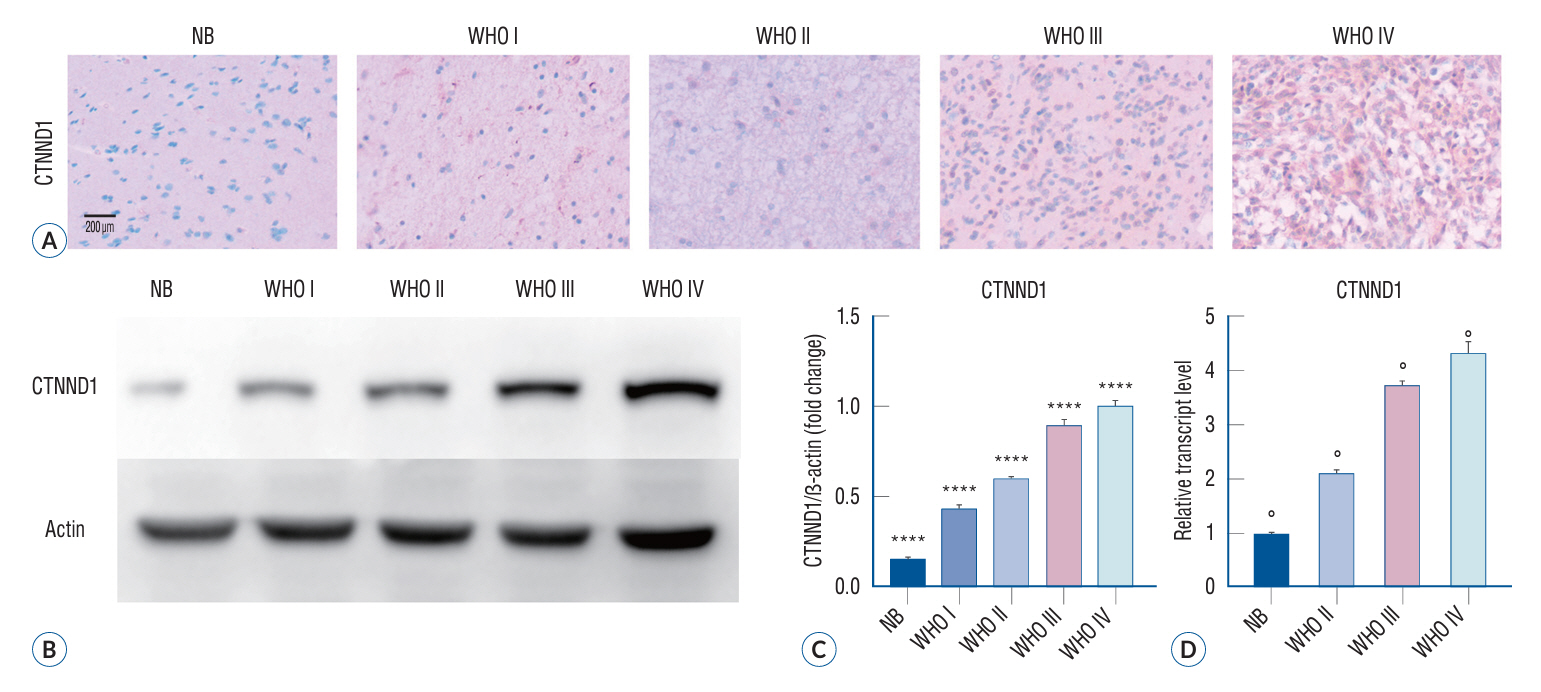
: Brain tissues of the glioma group exhibited a remarkable increase in the p120 expression level as compared to brain tissues of the nontumor group (p<0.05). Furthermore, a strong positive correlation was noted between the malignancy degree in glioma brain tissues and p120 expression in Western blotting (r=0.906, p<0.0001) and QT-PCR (F=830.6, p<0.01). Compared to the BC and NC groups, the siRNA transfection groups showed a significant suppression in p120 expression in glioma cells (p<0.05), with a marked attenuation in the invasive, migratory, and proliferative capabilities of glioma cells as well as an increase in apoptotic potential (p<0.05). Enzyme-labeled assay showed a remarkable increase in calcium concentration in glioma cells after siRNA treatment. Immunofluorescence assay revealed that the microtubule formation ability of glioma cells reduced after siRNA treatment.
Conclusion
: p120 has a pivotal involvement in facilitating glioma cell invasion and proliferation by potentially modulating these processes through its involvement in microtubule formation and regulation of intracellular calcium ion levels.
Keyword
Figure
Cited by 1 articles
-
Editors’ Pick in November 2024
Bum-Tae Kim
J Korean Neurosurg Soc. 2024;67(6):593-594. doi: 10.3340/jkns.2024.0190.
Reference
-
References
1. Barthel L, Hadamitzky M, Dammann P, Schedlowski M, Sure U, Thakur BK, et al. Glioma: molecular signature and crossroads with tumor microenvironment. Cancer Metastasis Rev. 41:53–75. 2022.
Article2. Becchetti A. Chapter two - interplay of Ca2+ and K+ signals in cell physiology and cancer. Curr Top Membr. 92:15–46. 2023.3. Boiarska Z, Passarella D. Microtubule-targeting agents and neurodegeneration. Drug Discov Today. 26:604–615. 2021.4. Di Cristofori A, Basso G, de Laurentis C, Mauri I, Sirtori MA, Ferrarese C, et al. Perspectives on (a)symmetry of arcuate fasciculus. A short review about anatomy, tractography and TMS for arcuate fasciculus reconstruction in planning surgery for gliomas in language areas. Front Neurol. 12:639822. 2021.5. Duñach M, Del Valle-Pérez B, García de Herreros A. p120-catenin in canonical Wnt signaling. Crit Rev Biochem Mol Biol. 52:327–339. 2017.
Article6. Goodson HV, Jonasson EM. Microtubules and microtubule-associated proteins. Cold Spring Harb Perspect Biol. 10:a022608. 2018.
Article7. Haag D, Zipper P, Westrich V, Karra D, Pfleger K, Toedt G, et al. Nos2 inactivation promotes the development of medulloblastoma in Ptch1(+/-) mice by deregulation of Gap43-dependent granule cell precursor migration. PLoS Genet. 8:e1002572. 2012.
Article8. Hatanaka K, Simons M, Murakami M. Phosphorylation of VE-cadherin controls endothelial phenotypes via p120-catenin coupling and Rac1 activation. Am J Physiol Heart Circ Physiol. 300:H162–H172. 2011.
Article9. Hernández A, Domènech M, Muñoz-Mármol AM, Carrato C, Balana C. Glioblastoma: relationship between metabolism and immunosuppressive microenvironment. Cells. 10:3529. 2021.
Article10. Hong JY, Oh IH, McCrea PD. Phosphorylation and isoform use in p120-catenin during development and tumorigenesis. Biochim Biophys Acta. 1863:102–114. 2016.
Article11. Jiang G, Wang Y, Dai S, Liu Y, Stoecker M, Wang E, et al. P120-catenin isoforms 1 and 3 regulate proliferation and cell cycle of lung cancer cells via β-catenin and Kaiso respectively. PLoS One. 7:e30303. 2012.
Article12. Jiang Y, Liao L, Shrestha C, Ji S, Chen Y, Peng J, et al. Reduced expression of E-cadherin and p120-catenin and elevated expression of PLC-γ1 and PIKE are associated with aggressiveness of oral squamous cell carcinoma. Int J Clin Exp Pathol. 8:9042–9051. 2015.13. Jung E, Osswald M, Ratliff M, Dogan H, Xie R, Weil S, et al. Tumor cell plasticity, heterogeneity, and resistance in crucial microenvironmental niches in glioma. Nat Commun. 12:1014. 2021.
Article14. Krigers A, Demetz M, Moser P, Kerschbaumer J, Brawanski KR, Fritsch H, et al. Impact of GAP-43, Cx43 and actin expression on the outcome and overall survival in diffuse and anaplastic gliomas. Sci Rep. 13:2024. 2023.
Article15. Latario CJ, Schoenfeld LW, Howarth CL, Pickrell LE, Begum F, Fischer DA, et al. Tumor microtubes connect pancreatic cancer cells in an Arp2/3 complex-dependent manner. Mol Biol Cell. 31:1259–1272. 2020.
Article16. Lee M, Ji H, Furuta Y, Park JI, McCrea PD. p120-catenin regulates REST and CoREST, and modulates mouse embryonic stem cell differentiation. J Cell Sci. 127(Pt 18):4037–4051. 2014.17. Lou E. A ticket to ride: the implications of direct intercellular communication via tunneling nanotubes in peritoneal and other invasive malignancies. Front Oncol. 10:559548. 2020.
Article18. Maiden SL, Petrova YI, Gumbiner BM. Microtubules inhibit E-cadherin adhesive activity by maintaining phosphorylated p120-catenin in a colon carcinoma cell model. PLoS One. 11:e0148574. 2016.
Article19. Osswald M, Jung E, Sahm F, Solecki G, Venkataramani V, Blaes J, et al. Brain tumour cells interconnect to a functional and resistant network. Nature. 528:93–98. 2015.
Article20. Osswald M, Solecki G, Wick W, Winkler F. A malignant cellular network in gliomas: potential clinical implications. Neuro Oncol. 18:479–485. 2016.
Article21. Parato J, Bartolini F. The microtubule cytoskeleton at the synapse. Neurosci Lett. 753:135850. 2021.
Article22. Poff A, Koutnik AP, Egan KM, Sahebjam S, D’Agostino D, Kumar NB. Targeting the Warburg effect for cancer treatment: ketogenic diets for management of glioma. Semin Cancer Biol. 56:135–148. 2019.
Article23. Portela M, Mitchell T, Casas-Tintó S. Cell-to-cell communication mediates glioblastoma progression in Drosophila. Biol Open. 9:bio053405. 2020.24. Przybylowski CJ, Hervey-Jumper SL, Sanai N. Surgical strategy for insular glioma. J Neurooncol. 51:491–497. 2021.
Article25. Roehlecke C, Schmidt MHH. Tunneling nanotubes and tumor microtubes in cancer. Cancers (Basel). 12:857. 2020.
Article26. Saberbaghi T, Wong R, Rutka JT, Wang GL, Feng ZP, Sun HS. Role of Cl-channels in primary brain tumour. Cell Calcium. 81:1–11. 2019.27. Venhuizen JH, Jacobs FJC, Span PN, Zegers MM. P120 and E-cadherin: double-edged swords in tumor metastasis. Semin Cancer Biol. 60:107–120. 2020.
Article28. Venkataramani V, Schneider M, Giordano FA, Kuner T, Wick W, Herrlinger U, et al. Disconnecting multicellular networks in brain tumours. Nat Rev Cancer. 22:481–491. 2022.
Article29. Venkataramani V, Yang Y, Schubert MC, Reyhan E, Tetzlaff SK, Wißmann N, et al. Glioblastoma hijacks neuronal mechanisms for brain invasion. Cell. 185:2899–2917.e31. 2022.
Article30. Venkatesh VS, Lou E. Tunneling nanotubes: a bridge for heterogeneity in glioblastoma and a new therapeutic target? Cancer Rep (Hoboken). 2:e1185. 2019.
Article31. Wang X, Liang J, Sun H. The network of tumor microtubes: an improperly reactivated neural cell network with stemness feature for resistance and recurrence in gliomas. Front Oncol. 12:921975. 2022.
Article32. Watson DC, Bayik D, Storevik S, Moreino SS, Sprowls SA, Han J, et al. GAP43-dependent mitochondria transfer from astrocytes enhances glioblastoma tumorigenicity. Nat Cancer. 4:648–664. 2023.
Article33. Weil S, Osswald M, Solecki G, Grosch J, Jung E, Lemke D, et al. Tumor microtubes convey resistance to surgical lesions and chemotherapy in gliomas. Neuro Oncol. 19:1316–1326. 2017.
Article34. Wu H, Yan X, Zhao L, Li X, Li X, Zhang Y, et al. p120-catenin promotes innate antiviral immunity through stabilizing TBK1-IRF3 complex. Mol Immunol. 157:8–17. 2023.
Article35. Xie R, Kessler T, Grosch J, Hai L, Venkataramani V, Huang L, et al. Tumor cell network integration in glioma represents a stemness feature. Neuro Oncol. 23:757–769. 2021.
Article36. Xie Z, Tang Y, Man MQ, Shrestha C, Bikle DD. p120-catenin is required for regulating epidermal proliferation, differentiation, and barrier function. J Cell Physiol. 234:427–432. 2018.
Article37. Xu S, Tang L, Li X, Fan F, Liu Z. Immunotherapy for glioma: current management and future application. Cancer Lett. 476:1–12. 2020.
Article38. Yasinjan F, Xing Y, Geng H, Guo R, Yang L, Liu Z, et al. Immunotherapy: a promising approach for glioma treatment. Front Immunol. 14:1255611. 2023.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Immunotherapeutic Approaches for Malignant Gliomas
- Inhibition of Wnt/ββ-catenin signaling by monensin in cervical cancer
- A Case of Brain Stem Glioma Responded to Tamoxifen after Relapse with Chemotherapy and Radiotherapy
- Myosin VI contributes to malignant proliferation of human glioma cells
- Impact of General Factors on Glioma Immunotherapy