Intest Res.
2024 Oct;22(4):473-483. 10.5217/ir.2023.00135.
Early change in serum leucine-rich α-2-glycoprotein predicts clinical and endoscopic response in ulcerative colitis
- Affiliations
-
- 1Center for Advanced IBD Research and Treatment, Kitasato University Kitasato Institute Hospital, Tokyo, Japan
- 2Department of Gastroenterology and Hepatology, Kitasato University Kitasato Institute Hospital, Tokyo, Japan
- 3Division of Gastroenterology and Hepatology, Toho University Omori Medical Center, Tokyo, Japan
- 4Department of Gastroenterology, Kitasato University School of Medicine, Sagamihara, Japan
- KMID: 2560297
- DOI: http://doi.org/10.5217/ir.2023.00135
Abstract
- Background/Aims
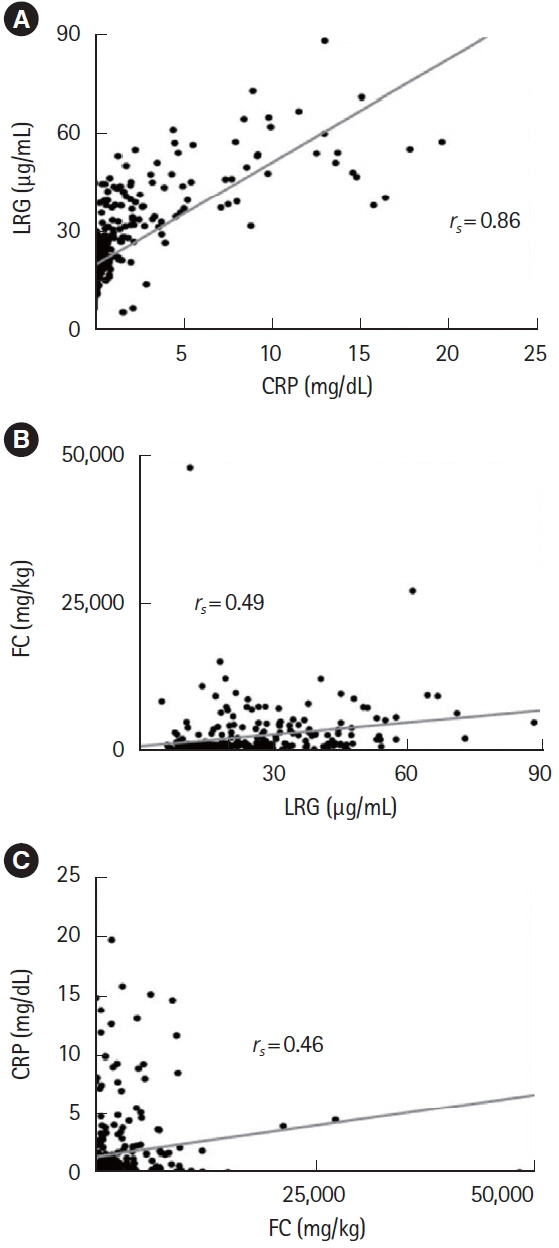
Leucine-rich α-2-glycoprotein (LRG) is a new serum biomarker reflecting the disease activity of ulcerative colitis (UC), but its change during the acute phase has not been enough investigated.
Methods
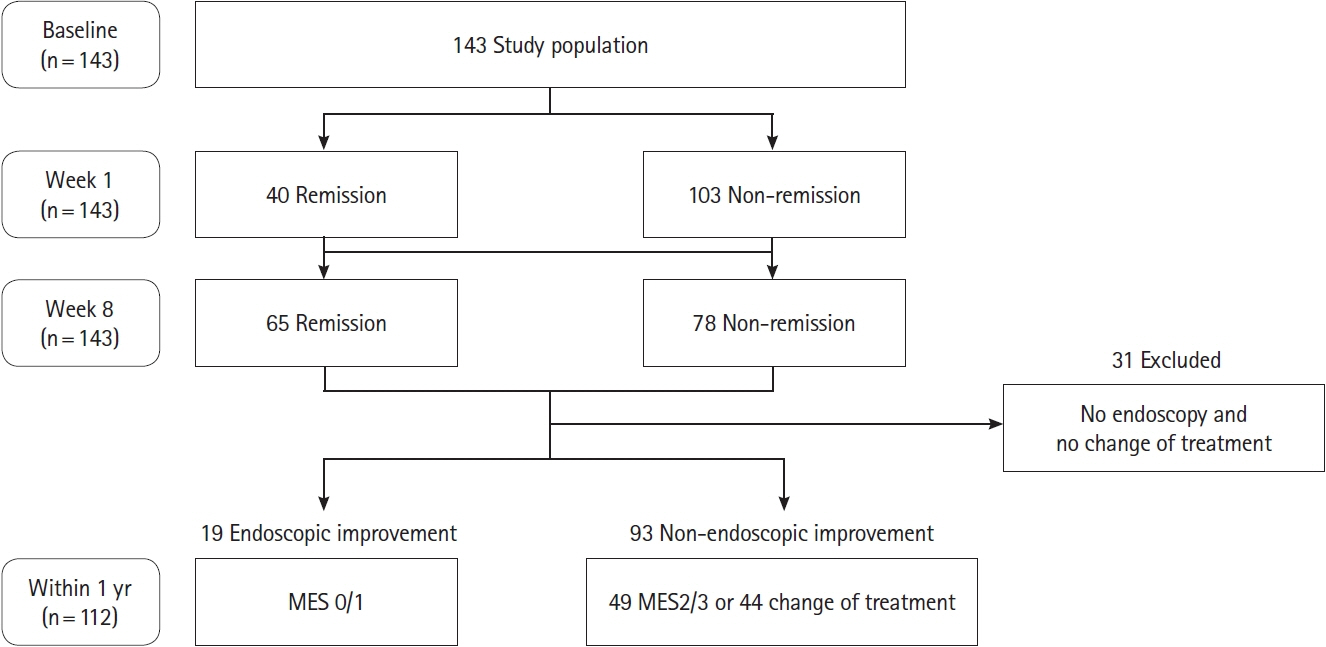
Patients with UC who initiated the induction therapy with steroid or advanced therapy (biologics or Janus kinase inhibitors) were prospectively enrolled. Associations of LRG, C-reactive protein (CRP) and fecal calprotectin (FC) at baseline, week 1, and week 8 with clinical remission at week 8 and subsequent endoscopic improvement within 1 year (Mayo endoscopic subscore of 0 or 1) were assessed.
Results
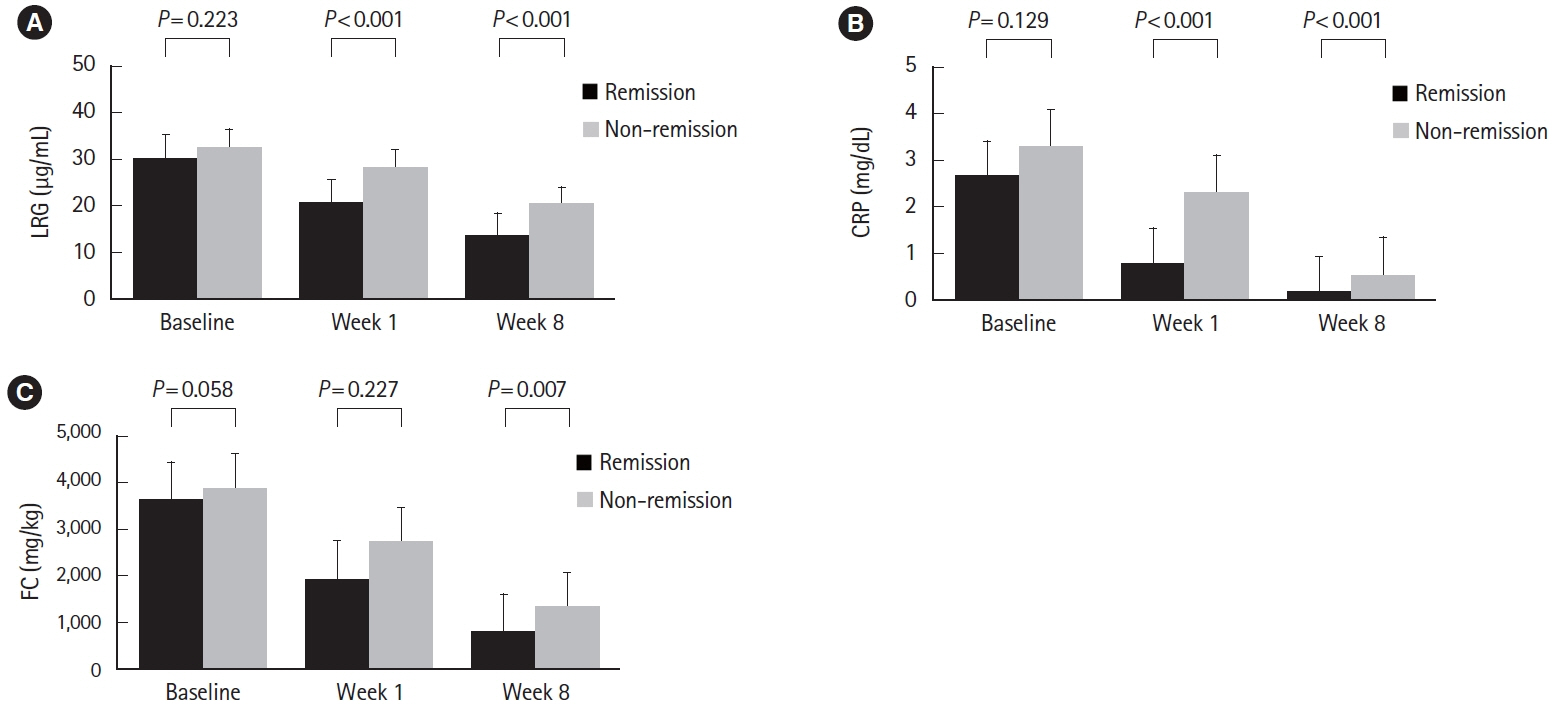
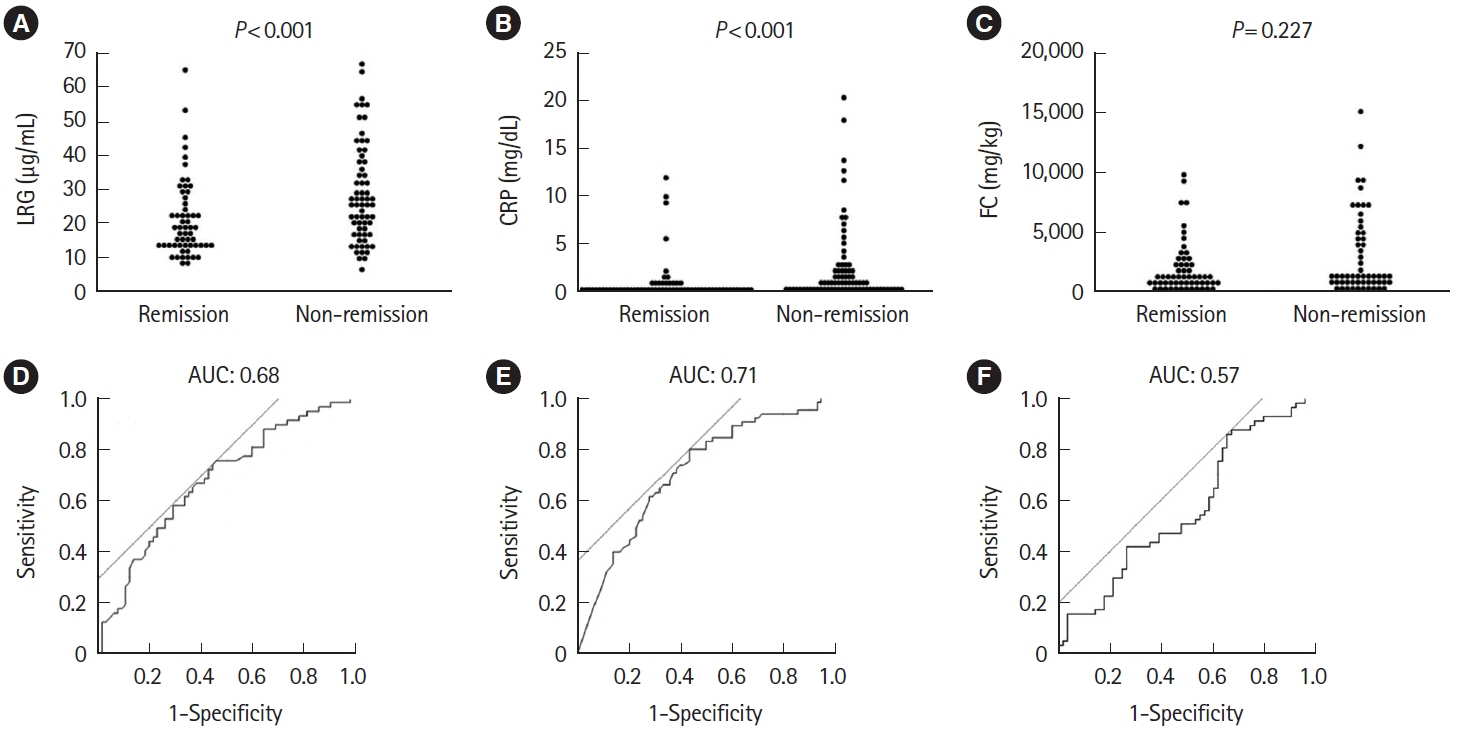
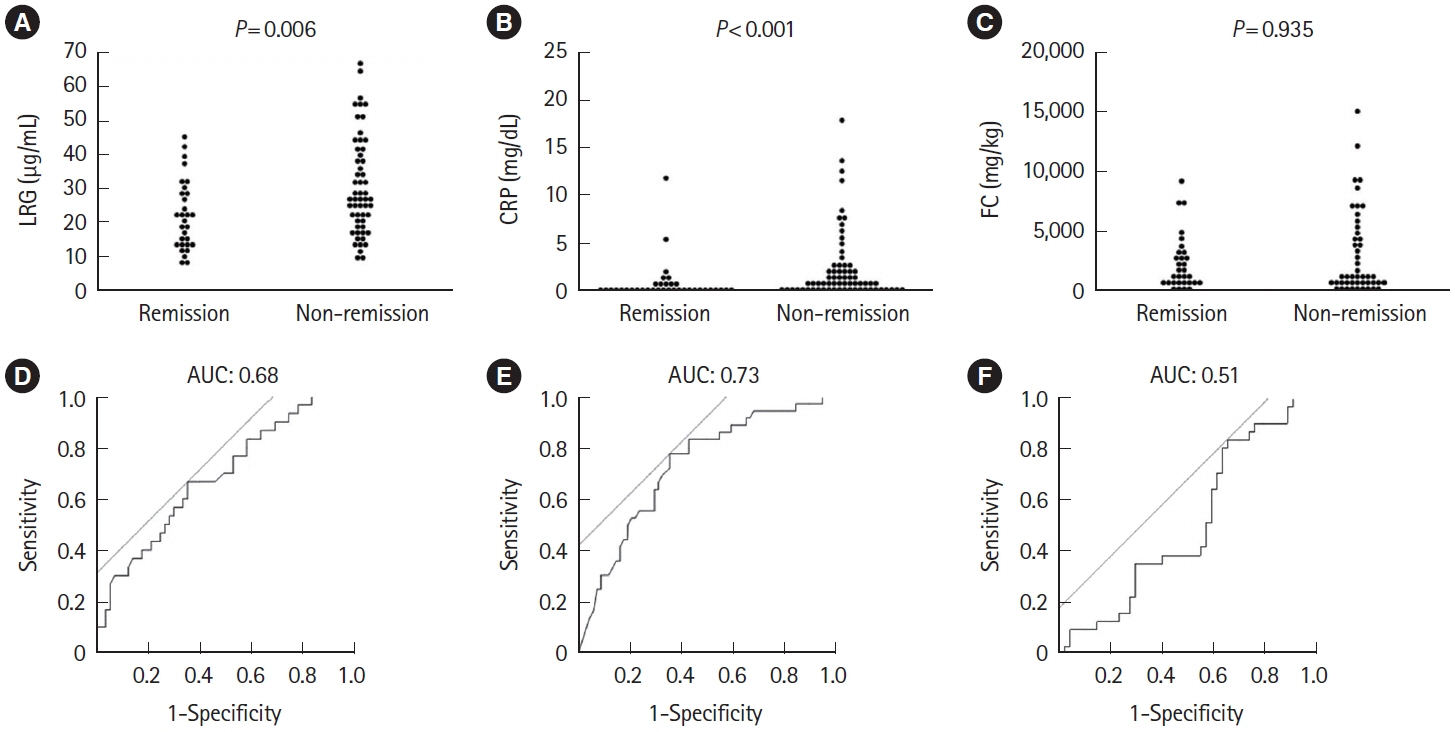
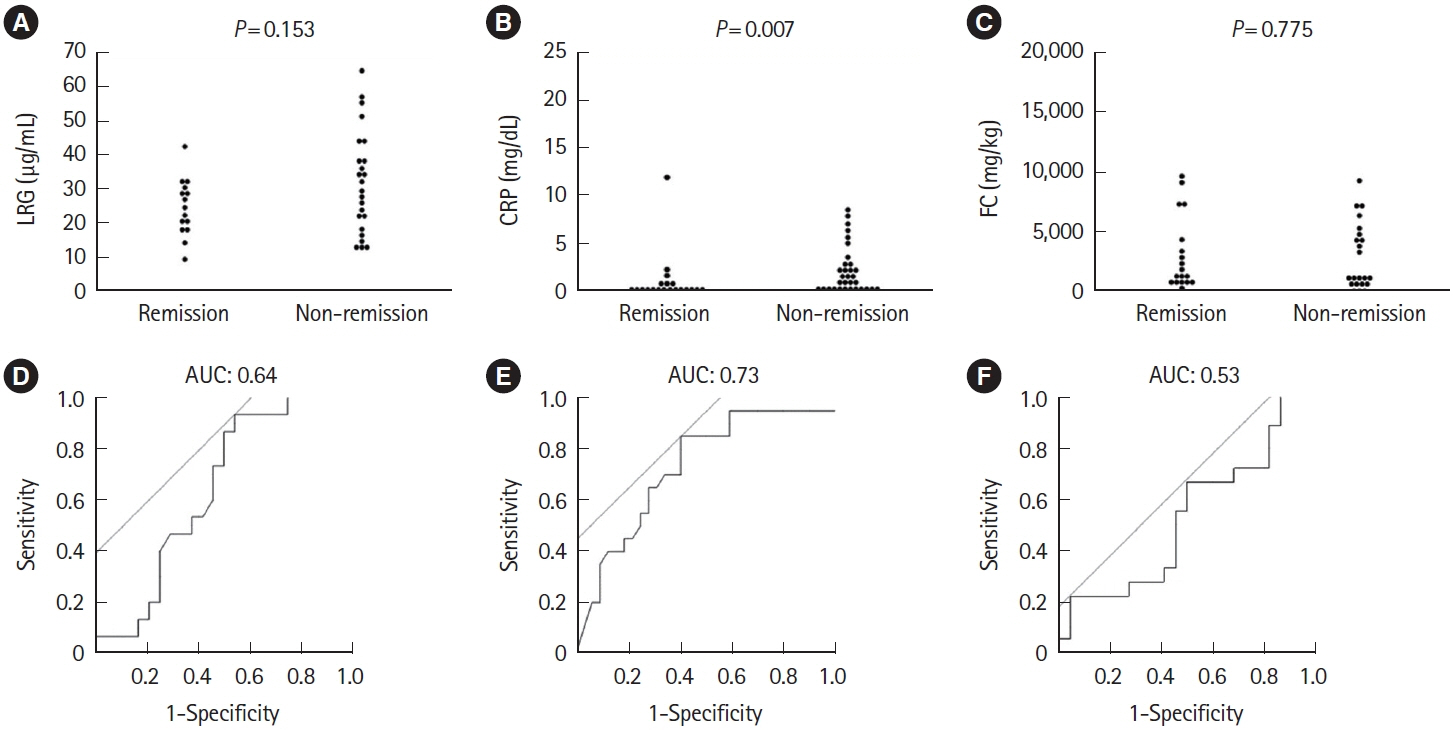
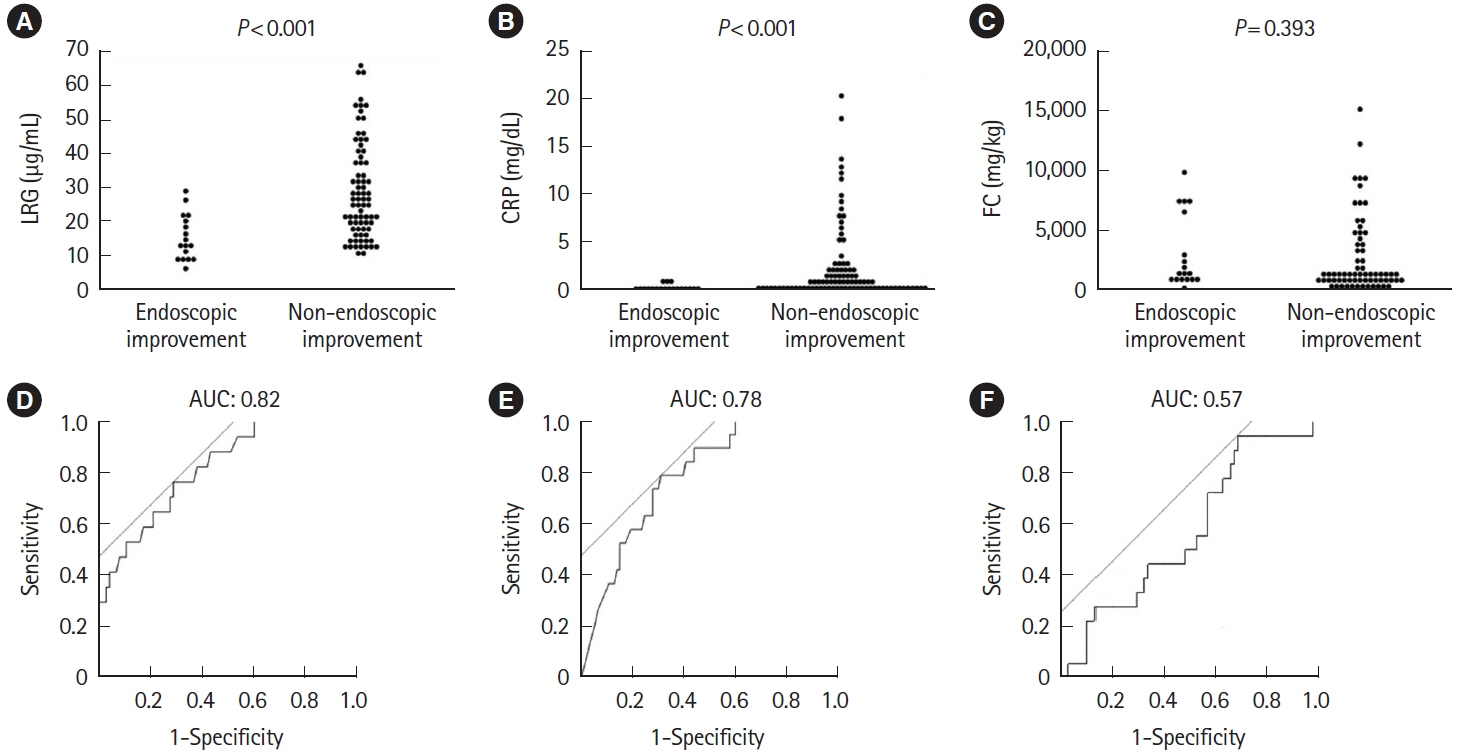
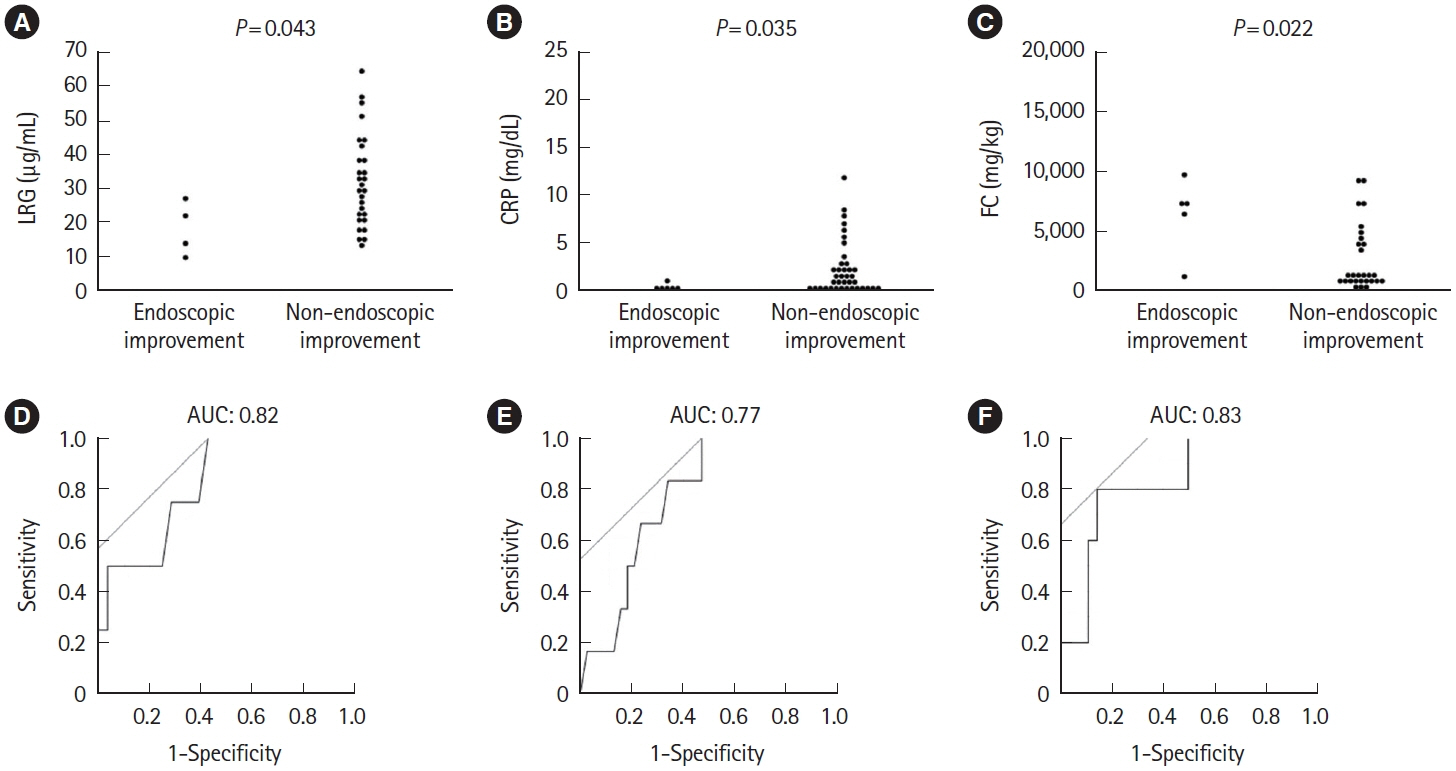
A total of 143 patients with UC were included. LRG and CRP at week 1 were significantly lower in the clinical remission group than in the non-remission group (LRG, 20.6 μg/mL vs. 28.4 μg/mL, P< 0.001; CRP, 0.9 mg/dL vs. 2.3 mg/dL, P< 0.001) while FC demonstrated the difference between groups only at week 8. The area under the curves of week 1 LRG, CRP, and FC for week 8 clinical remission using the receiver operating characteristic curves analysis were 0.68, 0.71, and 0.57, respectively. Furthermore, LRG and CRP predicted subsequent endoscopic improvement as early as week 1, while FC was predictive only at week 8.
Conclusions
LRG can be an early-phase biomarker predicting subsequent clinical and endoscopic response to induction therapy.
Figure
Reference
-
1. Kobayashi T, Siegmund B, Le Berre C, et al. Ulcerative colitis. Nat Rev Dis Primers. 2020; 6:74.2. Borren NZ, Khalili H, Luther J, Colizzo FP, Garber JJ, Ananthakrishnan AN. Second-look endoscopy in hospitalized severe ulcerative colitis: a retrospective cohort study. Inflamm Bowel Dis. 2019; 25:750–755.3. Kobayashi T, Naganuma M, Okamoto S, et al. Rapid endoscopic improvement is important for 1-year avoidance of colectomy but not for the long-term prognosis in cyclosporine A treatment for ulcerative colitis. J Gastroenterol. 2010; 45:1129–1137.4. Sagami S, Kobayashi T, Aihara K, et al. Transperineal ultrasound predicts endoscopic and histological healing in ulcerative colitis. Aliment Pharmacol Ther. 2020; 51:1373–1383.5. Kobayashi T, Suzuki Y, Motoya S, et al. First trough level of infliximab at week 2 predicts future outcomes of induction therapy in ulcerative colitis-results from a multicenter prospective randomized controlled trial and its post hoc analysis. J Gastroenterol. 2016; 51:241–251.6. Toyonaga T, Kobayashi T, Nakano M, et al. Usefulness of fecal calprotectin for the early prediction of short-term outcomes of remission-induction treatments in ulcerative colitis in comparison with two-item patient-reported outcome. PLoS One. 2017; 12:e0185131.7. Iwasa R, Yamada A, Sono K, Furukawa R, Takeuchi K, Suzuki Y. C-reactive protein level at 2 weeks following initiation of infliximab induction therapy predicts outcomes in patients with ulcerative colitis: a 3 year follow-up study. BMC Gastroenterol. 2015; 15:103.8. Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than the Lichtiger Index, C-reactive protein, platelets, hemoglobin, and blood leukocytes. Inflamm Bowel Dis. 2013; 19:332–341.9. Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008; 103:162–169.10. Yoon JY, Park SJ, Hong SP, Kim TI, Kim WH, Cheon JH. Correlations of C-reactive protein levels and erythrocyte sedimentation rates with endoscopic activity indices in patients with ulcerative colitis. Dig Dis Sci. 2014; 59:829–837.11. Ha YJ, Kang EJ, Lee SW, et al. Usefulness of serum leucine-rich alpha-2 glycoprotein as a disease activity biomarker in patients with rheumatoid arthritis. J Korean Med Sci. 2014; 29:1199–1204.12. Serada S, Fujimoto M, Terabe F, et al. Serum leucine-rich alpha-2 glycoprotein is a disease activity biomarker in ulcerative colitis. Inflamm Bowel Dis. 2012; 18:2169–2179.13. Shinzaki S, Matsuoka K, Iijima H, et al. Leucine-rich alpha-2 glycoprotein is a serum biomarker of mucosal healing in ulcerative colitis. J Crohns Colitis. 2017; 11:84–91.14. Shinzaki S, Matsuoka K, Tanaka H, et al. Leucine-rich alpha-2 glycoprotein is a potential biomarker to monitor disease activity in inflammatory bowel disease receiving adalimumab: PLANET study. J Gastroenterol. 2021; 56:560–569.15. Colombel JF, Keir ME, Scherl A, et al. Discrepancies between patient-reported outcomes, and endoscopic and histological appearance in UC. Gut. 2017; 66:2063–2068.16. D’Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007; 132:763–786.17. Sagami S, Kobayashi T, Aihara K, et al. Early improvement in bowel wall thickness on transperineal ultrasonography predicts treatment success in active ulcerative colitis. Aliment Pharmacol Ther. 2022; 55:1320–1329.18. Henriksen M, Jahnsen J, Lygren I, et al. C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease: results from a prospective population-based study. Gut. 2008; 57:1518–1523.19. Sandborn WJ, Panés J, Zhang H, Yu D, Niezychowski W, Su C. Correlation between concentrations of fecal calprotectin and outcomes of patients with ulcerative colitis in a phase 2 trial. Gastroenterology. 2016; 150:96–102.20. Zittan E, Kelly OB, Gralnek IM, Silverberg MS, Hillary Steinhart A. Fecal calprotectin correlates with active colonic inflammatory bowel disease but not with small intestinal Crohn’s disease activity. JGH Open. 2018; 2:201–206.21. D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012; 18:2218–2224.22. D’Incà R, Dal Pont E, Di Leo V, et al. Calprotectin and lactoferrin in the assessment of intestinal inflammation and organic disease. Int J Colorectal Dis. 2007; 22:429–437.23. Sjöwall C, Wetterö J. Pathogenic implications for autoantibodies against C-reactive protein and other acute phase proteins. Clin Chim Acta. 2007; 378:13–23.24. De Vos M, Dewit O, D’Haens G, et al. Fast and sharp decrease in calprotectin predicts remission by infliximab in anti-TNF naïve patients with ulcerative colitis. J Crohns Colitis. 2012; 6:557–562.25. Jain S, Kedia S, Bopanna S, et al. Faecal calprotectin and UCEIS predict short-term outcomes in acute severe colitis: prospective cohort study. J Crohns Colitis. 2017; 11:1309–1316.26. Lasson A, Stotzer PO, Öhman L, Isaksson S, Sapnara M, Strid H. The intra-individual variability of faecal calprotectin: a prospective study in patients with active ulcerative colitis. J Crohns Colitis. 2015; 9:26–32.27. Wagatsuma K, Yokoyama Y, Nakase H. Role of biomarkers in the diagnosis and treatment of inflammatory bowel disease. Life (Basel). 2021; 11:1375.28. Du L, Foshaug R, Huang VW, et al. Within-stool and withinday sample variability of fecal calprotectin in patients with inflammatory bowel disease: a prospective observational study. J Clin Gastroenterol. 2018; 52:235–240.29. Dubinsky MC, Magro F, Steinwurz F, et al. Association of C-reactive protein and partial Mayo score with response to tofacitinib induction therapy: results from the ulcerative colitis clinical program. Inflamm Bowel Dis. 2023; 29:51–61.30. Lindgren SC, Flood LM, Kilander AF, Löfberg R, Persson TB, Sjödahl RI. Early predictors of glucocorticosteroid treatment failure in severe and moderately severe attacks of ulcerative colitis. Eur J Gastroenterol Hepatol. 1998; 10:831–835.31. Feuerstein JD, Isaacs KL, Schneider Y, et al. AGA Clinical Practice Guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology. 2020; 158:1450–1461.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pathophysiology of ulcerative colitis - Relationship with genetics and immunity
- Malignant change of chronic ulcerative colitis : report of a case
- A Case of Malignant Lymphoma in Patient with Ulcerative Colitis
- Combined eosinophilic gastroenteritis and ulcerative colitis successfully treated by vedolizumab: a case report
- A case of ulcerative colitis