Cancer Res Treat.
2024 Oct;56(4):1270-1276. 10.4143/crt.2023.1278.
Combination of Dabrafenib and Trametinib in Patients with Metastatic BRAFV600E-Mutated Thyroid Cancer
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Division of Endocrinology and Metabolism, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2560260
- DOI: http://doi.org/10.4143/crt.2023.1278
Abstract
- Purpose
BRAF mutations are detected in 30%-80% of papillary thyroid cancer (PTC) cases. DaBRAFenib and trametinib showed promising antitumor activity in patients with BRAFV600E-mutated metastatic melanoma and non–small cell lung cancer. This study aimed to evaluate the efficacy and safety of daBRAFenib and trametinib in patients with metastatic BRAFV600E-mutated thyroid cancer.
Materials and Methods
This was a retrospective study to evaluate the efficacy of daBRAFenib and trametinib in patients with metastatic BRAFV600E-mutated PTC. The patients received daBRAFenib 150 mg twice daily and trametinib 2 mg once daily at the Samsung Medical Center. This study evaluated the progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR) overall survival (OS), and safety of daBRAFenib and trametinib.
Results
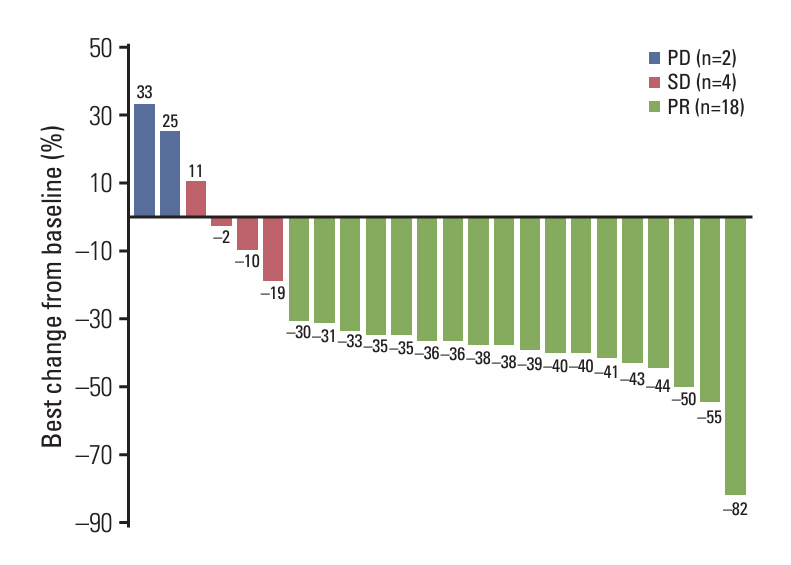
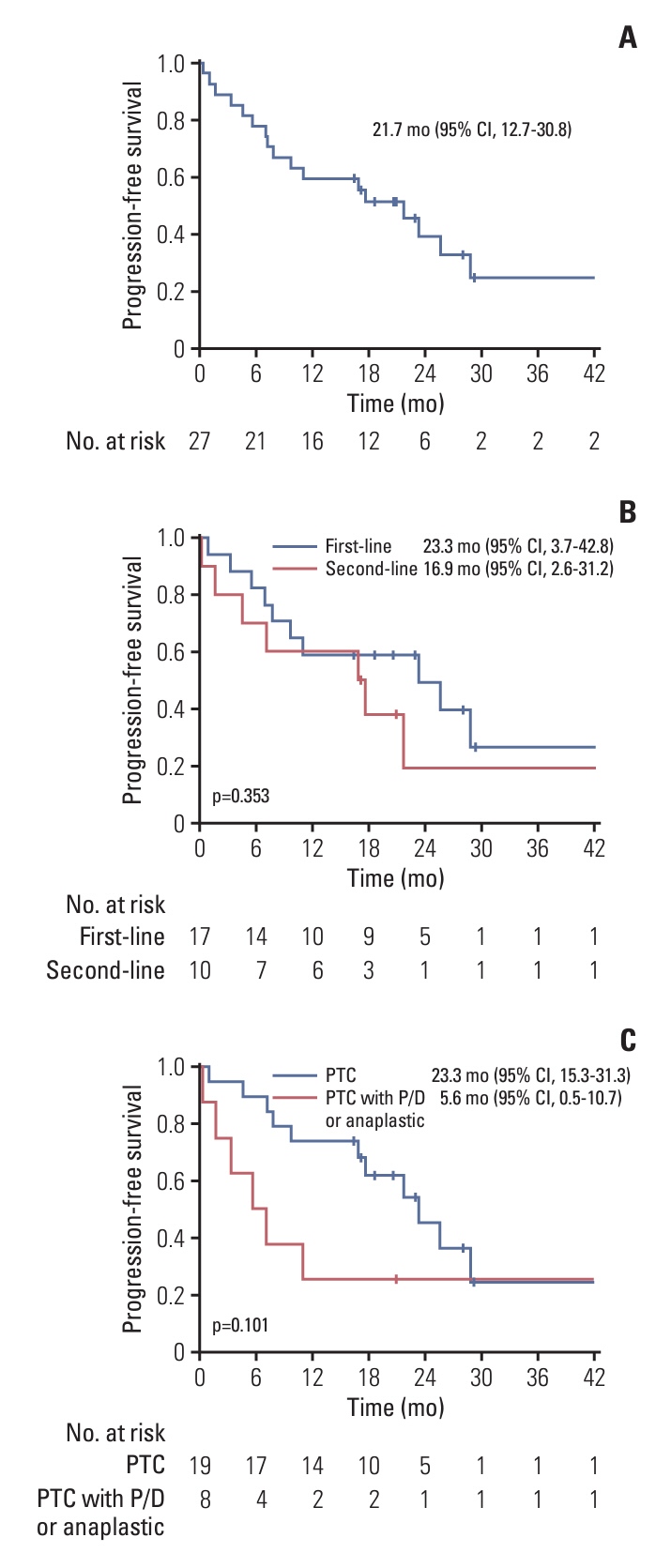
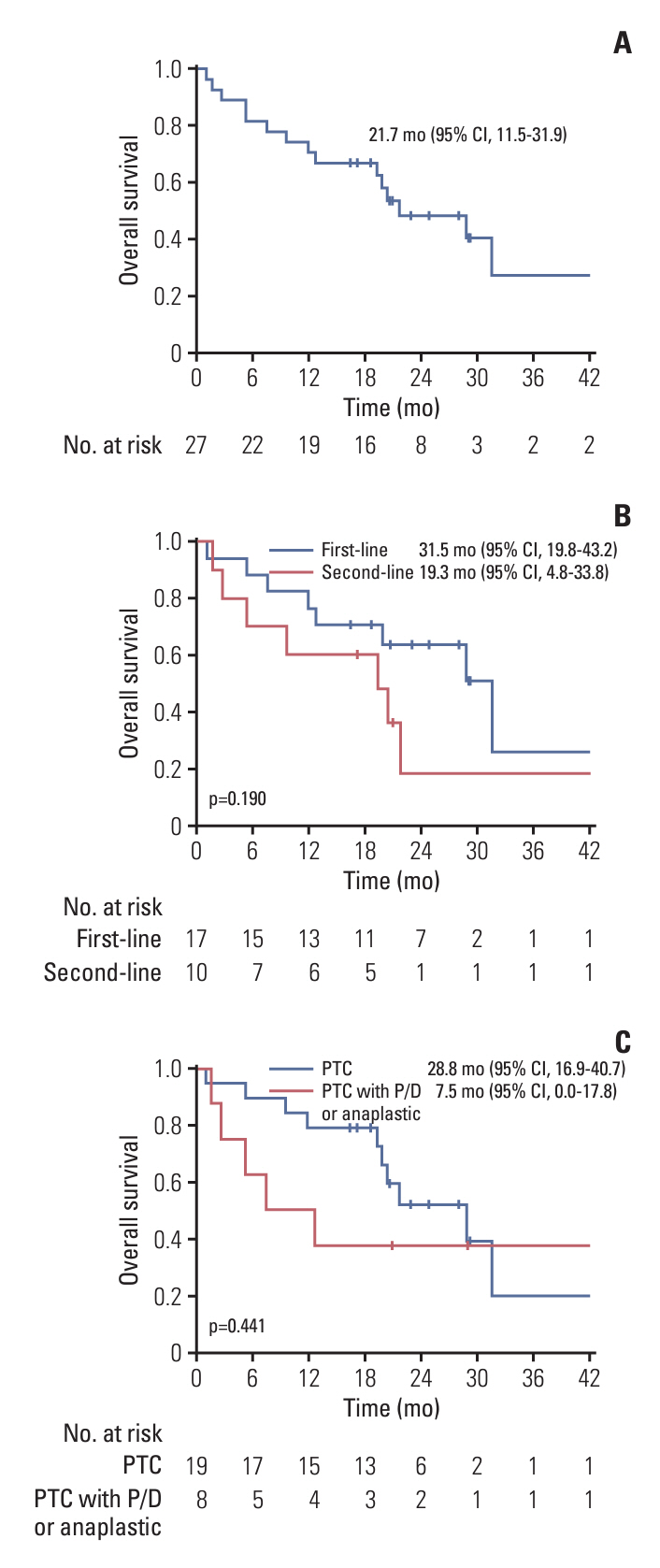
Between December 2019 and January 2022, 27 PTC patients including eight patients with poorly differentiated or anaplastic transformation, received daBRAFenib and trametinib. The median age was 73.0 years, and the median follow-up period was 19.8 months. The majority (81.5%) had undergone thyroidectomy, while 8 patients had received prior systemic treatments. ORR was 73.1%, with 19 partial responses, and DCR was 92.3%. Median PFS was 21.7 months, and median OS was 21.7 months. Treatment-related adverse events included generalized weakness (29.6%), fever (25.9%), and gastrointestinal problems (22.2%). Dose reduction due to adverse events was required in 81.5% of the patients.
Conclusion
DaBRAFenib and trametinib demonstrated a high ORR with promising PFS; however, most patients with BRAFV600E-mutated metastatic PTC required a dose reduction.
Keyword
Figure
Cited by 1 articles
-
Korean Thyroid Association Guidelines on the Management of Differentiated Thyroid Cancers; Part III. Management of Advanced Differentiated Thyroid Cancers - Chapter 4. Systemic Therapy for Progressive Radioiodine-Refractory Differentiated Thyroid Cancer 2024
Dong Yeob Shin, Ho-Cheol Kang, Sun Wook Kim, Dong Gyu Na, Young Joo Park, Young Shin Song, Eun Kyung Lee, Dong-Jun Lim, Yun Jae Chung, Won Gu Kim
Int J Thyroidol. 2024;17(1):168-181. doi: 10.11106/ijt.2024.17.1.168.
Reference
-
References
1. Miranda-Filho A, Lortet-Tieulent J, Bray F, Cao B, Franceschi S, Vaccarella S, et al. Thyroid cancer incidence trends by histology in 25 countries: a population-based study. Lancet Diabetes Endocrinol. 2021; 9:225–34.
Article2. Kim J, Gosnell JE, Roman SA. Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol. 2020; 16:17–29.
Article3. Sherman SI. Thyroid carcinoma. Lancet. 2003; 361:501–11.
Article4. Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, et al. SEER cancer statistics review, 1975-2015, based on November 2017 SEER data submission, posted to the SEER web site [Internet]. Bethesda, MD: National Cancer Institute;2018. [cited 2023 Jan 30]. Available from: https://seer.cancer.gov/csr/1975_2015/.5. Brito JP, Hay ID, Morris JC. Low risk papillary thyroid cancer. BMJ. 2014; 348:g3045.
Article6. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994; 97:418–28.
Article7. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016; 26:1–133.
Article8. National Comprehensive Cancer Network. Thyroid carcinoma (version 3.2022) [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network;2023. [cited 2023 Jan 30]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf.9. Ragazzi M, Ciarrocchi A, Sancisi V, Gandolfi G, Bisagni A, Piana S. Update on anaplastic thyroid carcinoma: morphological, molecular, and genetic features of the most aggressive thyroid cancer. Int J Endocrinol. 2014; 2014:790834.
Article10. Fagin JA, Wells SA Jr. Biologic and clinical perspectives on thyroid cancer. N Engl J Med. 2016; 375:1054–67.
Article11. Saini S, Tulla K, Maker AV, Burman KD, Prabhakar BS. Therapeutic advances in anaplastic thyroid cancer: a current perspective. Mol Cancer. 2018; 17:154.
Article12. Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, et al. Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol. 2022; 33:27–63.
Article13. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015; 372:621–30.
Article14. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014; 384:319–28.
Article15. Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest. 2016; 126:1052–66.
Article16. Peyssonnaux C, Eychene A. The Raf/MEK/ERK pathway: new concepts of activation. Biol Cell. 2001; 93:53–62.
Article17. Liu D, Liu Z, Condouris S, Xing M. BRAF V600E maintains proliferation, transformation, and tumorigenicity of BRAF-mutant papillary thyroid cancer cells. J Clin Endocrinol Metab. 2007; 92:2264–71.
Article18. King AJ, Arnone MR, Bleam MR, Moss KG, Yang J, Fedorowicz KE, et al. Dabrafenib; preclinical characterization, increased efficacy when combined with trametinib, while BRAF/MEK tool combination reduced skin lesions. PLoS One. 2013; 8:e67583.
Article19. Kim KB, Kefford R, Pavlick AC, Infante JR, Ribas A, Sosman JA, et al. Phase II study of the MEK1/MEK2 inhibitor trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol. 2013; 31:482–9.20. Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014; 371:1877–88.
Article21. Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015; 386:444–51.
Article22. Planchard D, Smit EF, Groen HJ, Mazieres J, Besse B, Helland A, et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. 2017; 18:1307–16.
Article23. Planchard D, Besse B, Groen HJ, Hashemi SM, Mazieres J, Kim TM, et al. Phase 2 study of dabrafenib plus trametinib in patients with BRAF V600E-mutant metastatic NSCLC: updated 5-year survival rates and genomic analysis. J Thorac Oncol. 2022; 17:103–15.
Article24. Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol. 2018; 36:7–13.
Article25. Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, et al. Dabrafenib plus trametinib in patients with BRAF V600E-mutant anaplastic thyroid cancer: updated analysis from the phase II ROAR basket study. Ann Oncol. 2022; 33:406–15.
Article26. Busaidy NL, Konda B, Wei L, Wirth LJ, Devine C, Daniels GA, et al. Dabrafenib versus dabrafenib + trametinib in BRAF-mutated radioactive iodine refractory differentiated thyroid cancer: results of a randomized, phase 2, open-label multicenter trial. Thyroid. 2022; 32:1184–92.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- BRAF/ MEK Inhibitors in Downstaging BRAFV600E Mutated Papillary Thyroid Cancer to Allow Resection: Case Report and Literature Review
- Augmentation of Radioiodine Uptake by Pulmonary Metastasis of Papillary Thyroid Carcinoma Treated with Dabrafenib and Trametinib
- Effectiveness and Safety of Dabrafenib in the Treatment of 20 Chinese Children with BRAFV600E-Mutated Langerhans Cell Histiocytosis
- Treatment Effect of Combining Lenvatinib and Vemurafenib for BRAF Mutated Anaplastic Thyroid Cancer
- Clinical Implication of BRAF Mutation in Thyroid Cancer