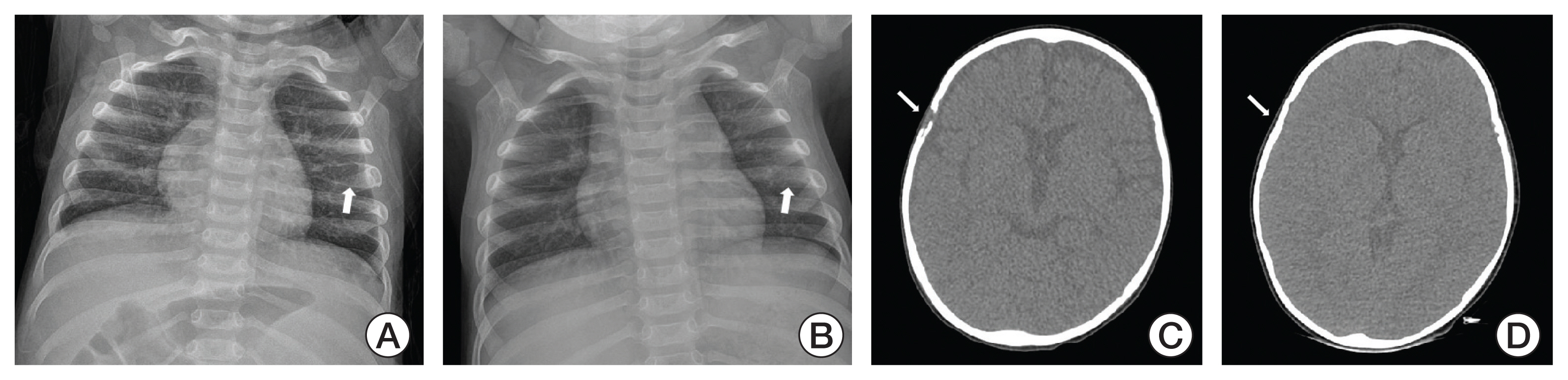
Cancer Res Treat.
2021 Jan;53(1):261-269. 10.4143/crt.2020.769.
Effectiveness and Safety of Dabrafenib in the Treatment of 20 Chinese Children with BRAFV600E-Mutated Langerhans Cell Histiocytosis
- Affiliations
-
- 1Beijing Key Laboratory of Pediatric Hematology Oncology; National Key Discipline of Pediatrics, Capital Medical University; Key Laboratory of Major Diseases in Children, Ministry of Education; Hematology Oncology Center, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, China
- 2Laboratory of Hematologic Diseases, Beijing Pediatric Research Institute, Beijing Children's Hospital, Capital Medical University, National Center for Children’s Health, Beijing, China
- KMID: 2510668
- DOI: http://doi.org/10.4143/crt.2020.769
Abstract
- Purpose
We sought to investigate the effectiveness and safety of dabrafenib in children with BRAFV600E-mutated Langerhans cell histiocytosis (LCH).
Materials and Methods
A retrospective analysis was performed on 20 children with BRAFV600E-mutated LCH who were treated with dabrafenib.
Results
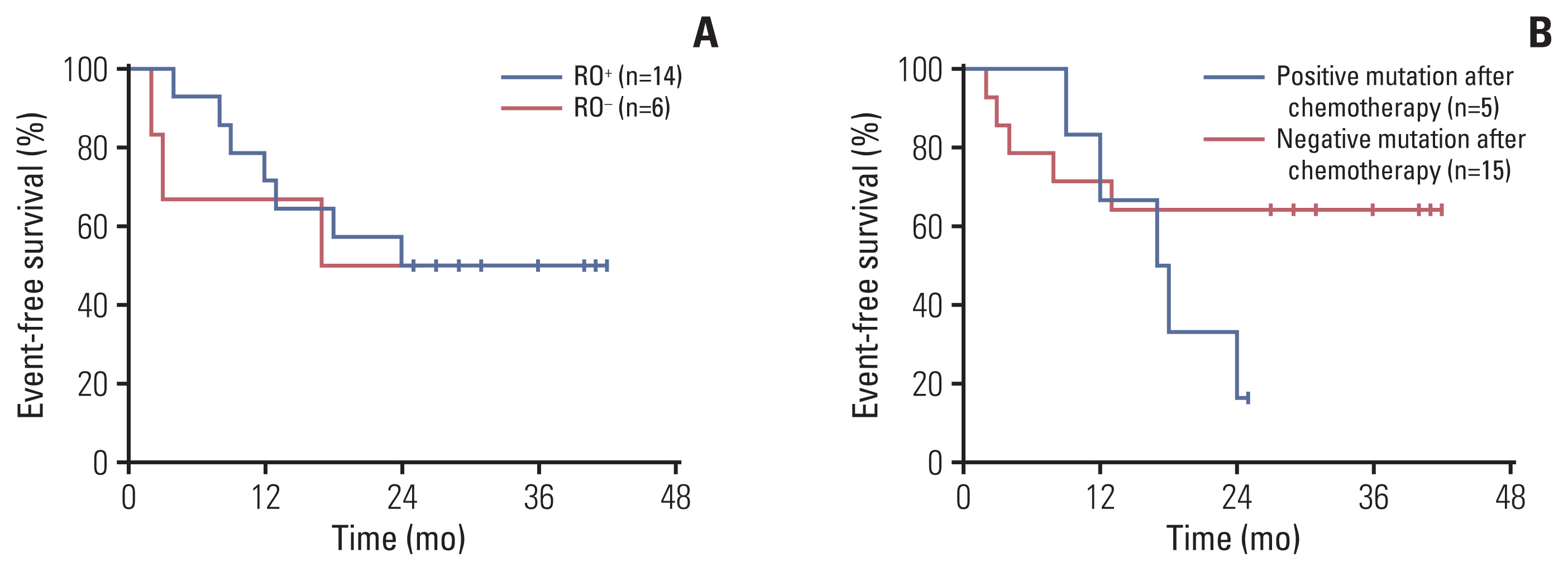
The median age at which the patients started taking dabrafenib was 2.3 years old (range, 0.6 to 6.5 years). The ratio of boys to girls was 2.3:1. The median follow-up time was 30.8 months (range, 18.9 to 43.6 months). There were 14 patients (70%) in the risk organ (RO)+ group and six patients (30%) in the RO– group. All patients were initially treated with traditional chemotherapy and then shifted to targeted therapy due to poor control of LCH or intolerance to chemotherapy. The overall objective response rate and the overall disease control rate were 65% and 75%, respectively. During treatment, circulating levels of cell-free BRAFV600E (cfBRAFV600E) became negative in 60% of the patients within a median period of 3.0 months (range, 1.0 to 9.0 months). Grade 2 or 3 adverse effects occurred in five patients.
Conclusion
Some children with BRAFV600E-mutated LCH may benefit from monotherapy with dabrafenib, especially high-risk patients with concomitant hemophagocytic lymphohistiocytosis and intolerance to chemotherapy. The safety of dabrafenib is notable. A prospective study with a larger sample size is required to determine the optimal dosage and treatment duration.
Keyword
Figure
Cited by 2 articles
-
Recent advances in the understanding of the molecular pathogenesis and targeted therapy options in Langerhans cell histiocytosis
Jin Kyung Suh, Sunghan Kang, Hyery Kim, Ho Joon Im, Kyung-Nam Koh
Blood Res. 2021;56(S1):65-69. doi: 10.5045/br.2021.2021013.Advancements in the understanding and management of histiocytic neoplasms
Kyung‑Nam Koh, Su Hyun Yoon, Sung Han Kang, Hyery Kim, Ho Joon Im
Blood Res. 2024;59:22. doi: 10.1007/s44313-024-00022-w.
Reference
-
References
1. Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med. 2018; 379:856–68.
Article2. Tran G, Huynh TN, Paller AS. Langerhans cell histiocytosis: a neoplastic disorder driven by Ras-ERK pathway mutations. J Am Acad Dermatol. 2018; 78:579–90.
Article3. Heritier S, Jehanne M, Leverger G, Emile JF, Alvarez JC, Haroche J, et al. Vemurafenib use in an infant for high-risk Langerhans cell histiocytosis. JAMA Oncol. 2015; 1:836–8.
Article4. Heritier S, Emile JF, Barkaoui MA, Thomas C, Fraitag S, Boudjemaa S, et al. BRAF mutation correlates with high-risk Langerhans cell histiocytosis and increased resistance to first-line therapy. J Clin Oncol. 2016; 34:3023–30.5. Badalian-Very G, Vergilio JA, Degar BA, MacConaill LE, Brandner B, Calicchio ML, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010; 116:1919–23.
Article6. Abla O, Weitzman S. Treatment of Langerhans cell histiocytosis: role of BRAF/MAPK inhibition. Hematology Am Soc Hematol Educ Program. 2015; 2015:565–70.
Article7. Cebollero A, Puertolas T, Pajares I, Calera L, Anton A. Comparative safety of BRAF and MEK inhibitors (vemurafenib, dabrafenib and trametinib) in first-line therapy for BRAF-mutated metastatic melanoma. Mol Clin Oncol. 2016; 5:458–62.
Article8. Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011; 364:2507–16.
Article9. Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012; 380:358–65.
Article10. Hertzman Johansson C, Egyhazi Brage S. BRAF inhibitors in cancer therapy. Pharmacol Ther. 2014; 142:176–82.
Article11. Kieran MW, Geoerger B, Dunkel IJ, Broniscer A, Hargrave D, Hingorani P, et al. A phase I and pharmacokinetic study of oral dabrafenib in children and adolescent patients with recurrent or refractory BRAF V600 mutation-positive solid tumors. Clin Cancer Res. 2019; 25:7294–302.
Article12. Toll SA, Tran HN, Cotter J, Judkins AR, Tamrazi B, Biegel JA, et al. Sustained response of three pediatric BRAF(V600E) mutated high-grade gliomas to combined BRAF and MEK inhibitor therapy. Oncotarget. 2019; 10:551–7.
Article13. Wang D, Cui L, Li ZG, Zhang L, Lian HY, Ma HH, et al. Clinical research of pulmonary Langerhans cell histiocytosis in children. Chin Med J (Engl). 2018; 131:1793–8.
Article14. LCH evaluation and treatment guidelines [Internet]. Pitman NJ: Histiocyte Society;2009. [cited 2020 Sep 14]. Available from: https://histiocytesociety.org/document.doc?id=290 .15. Common Terminology Criteria for Adverse Events (CTCAE) [Internet]. Bethesda, MD: National Cancer Institute;2017. [cited 2020 Sep 14]. Available from: https://ctep.cancer.gov/ .16. Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007; 48:124–31.
Article17. Ozer E, Sevinc A, Ince D, Yuzuguldu R, Olgun N. BRAF V600E mutation: a significant biomarker for prediction of disease relapse in pediatric Langerhans cell histiocytosis. Pediatr Dev Pathol. 2019; 22:449–55.18. Heritier S, Helias-Rodzewicz Z, Lapillonne H, Terrones N, Garrigou S, Normand C, et al. Circulating cell-free BRAF(V600E) as a biomarker in children with Langerhans cell histiocytosis. Br J Haematol. 2017; 178:457–67.19. Schadendorf D, Hauschild A, Santinami M, Atkinson V, Mandala M, Chiarion-Sileni V, et al. Patient-reported outcomes in patients with resected, high-risk melanoma with BRAF(V600E) or BRAF(V600K) mutations treated with adjuvant dabrafenib plus trametinib (COMBI-AD): a randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2019; 20:701–10.
Article20. Awada G, Seremet T, Fostier K, Everaert H, Neyns B. Long-term disease control of Langerhans cell histiocytosis using combined BRAF and MEK inhibition. Blood Adv. 2018; 2:2156–8.
Article21. Mourah S, Lorillon G, Meignin V, Vercellino L, de Margerie-Mellon C, Pages C, et al. Dramatic transient improvement of metastatic BRAF(V600E)-mutated Langerhans cell sarcoma under treatment with dabrafenib. Blood. 2015; 126:2649–52.
Article22. Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay JY, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015; 373:726–36.
Article23. McClain KL, Picarsic J, Chakraborty R, Zinn D, Lin H, Abhyankar H, et al. CNS Langerhans cell histiocytosis: common hematopoietic origin for LCH-associated neurodegeneration and mass lesions. Cancer. 2018; 124:2607–20.
Article24. Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015; 372:30–9.
Article25. Yamazaki N, Tsutsumida A, Takahashi A, Namikawa K, Yoshikawa S, Fujiwara Y, et al. Phase 1/2 study assessing the safety and efficacy of dabrafenib and trametinib combination therapy in Japanese patients with BRAF V600 mutation-positive advanced cutaneous melanoma. J Dermatol. 2018; 45:397–407.
Article26. Davies MA, Saiag P, Robert C, Grob JJ, Flaherty KT, Arance A, et al. Dabrafenib plus trametinib in patients with BRAF(V600)-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017; 18:863–73.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A study of pathological characteristics and BRAF V600E status in Langerhans cell histiocytosis of Vietnamese children
- Combination of Dabrafenib and Trametinib in Patients with Metastatic BRAFV600E-Mutated Thyroid Cancer
- Selection Strategies and Practical Application of BRAF V600E-Mutated Non–Small Cell Lung Carcinoma
- Improvement of Neurodegenerative Disease after Use of Vemurafenib in Refractory BRAF V600E-Mutated Langerhans Cell Histiocytosis: A Case Report
- BRAF/ MEK Inhibitors in Downstaging BRAFV600E Mutated Papillary Thyroid Cancer to Allow Resection: Case Report and Literature Review