Cancer Res Treat.
2024 Oct;56(4):1231-1239. 10.4143/crt.2024.237.
Distinct Characteristics and Changes in Liver Function of Patients with Hepatocellular Carcinoma Treated with Atezolizumab Plus Bevacizumab for More Than 1 Year
- Affiliations
-
- 1CHA University School of Medicine, Seongnam, Korea
- 2Division of Medical Oncology, Department of Internal Medicine, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea
- 3Division of Oncology, Department of Internal Medicine, Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
- 4Department of Internal Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea
- 5Department of Radiology, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea
- KMID: 2560256
- DOI: http://doi.org/10.4143/crt.2024.237
Abstract
- Purpose
Since 2020, atezolizumab plus bevacizumab (Ate/Bev) has been the standard first-line therapy for unresectable hepatocellular carcinoma (HCC), but long-term treatment studies are limited. This study evaluated the clinical characteristics and effects of Ate/Bev for over 1 year.
Materials and Methods
This study included patients with unresectable HCC treated with first-line Ate/Bev between May 2020 and April 2022. Those receiving Ate/Bev for 1 year or more were classified as the long-term treatment group.
Results
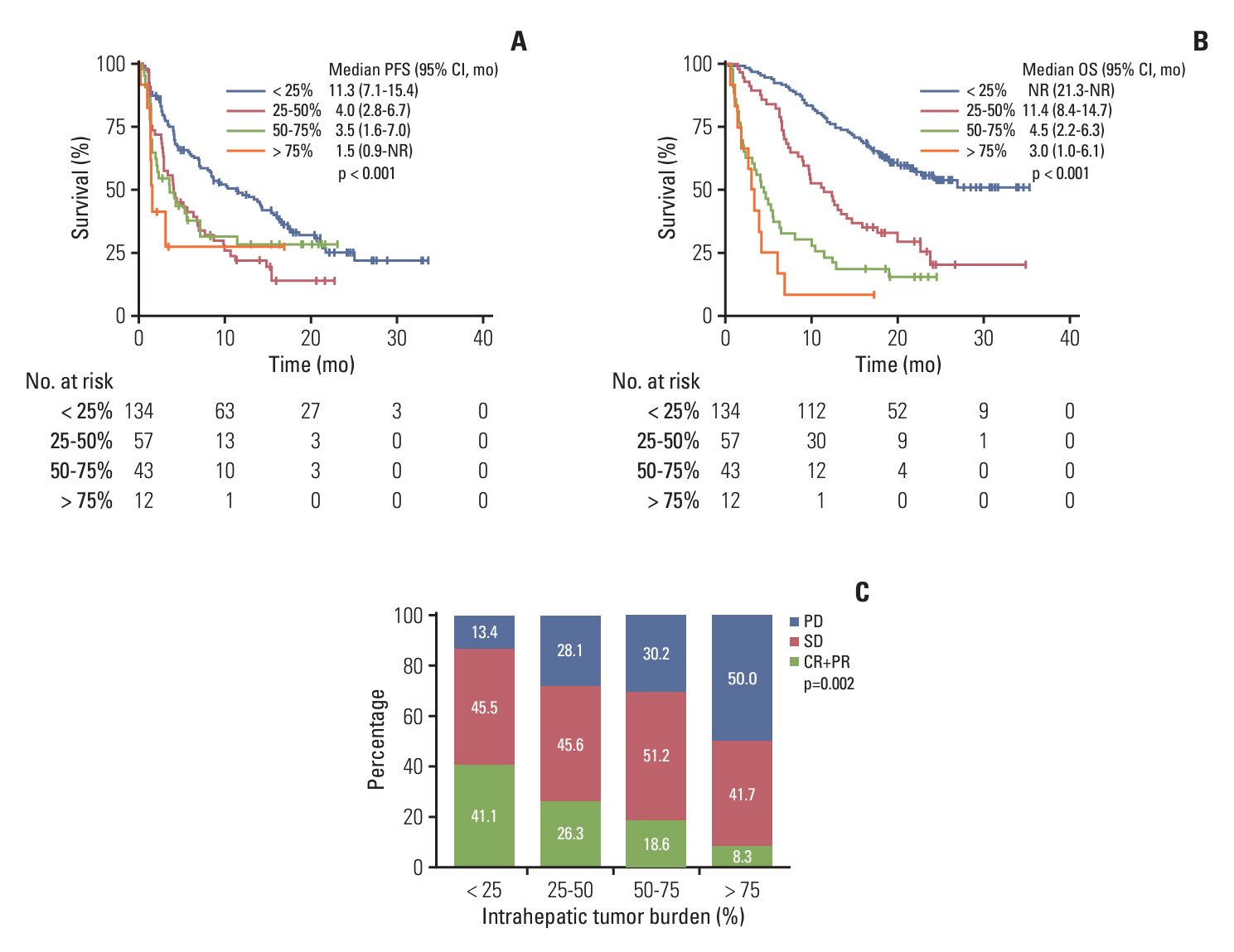
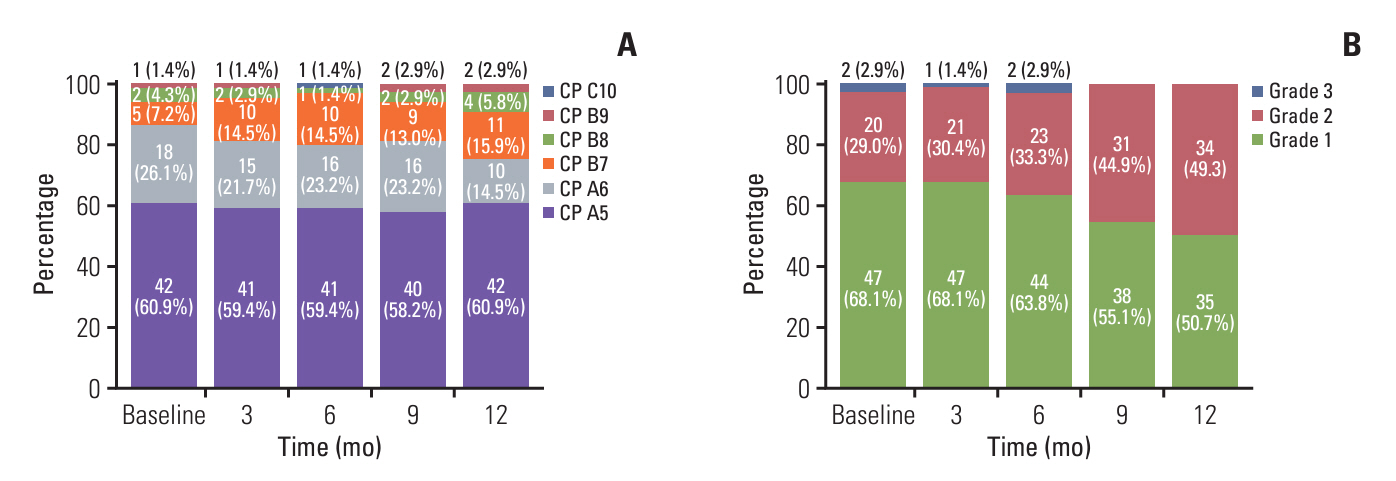
Of 246 patients, 69 (28.0%) were in the long-term treatment group, which comprised more proportions of intrahepatic tumor burden < 25%, Eastern Cooperative Oncology Group 0, and a lower proportion of portal vein tumor thrombosis than the short-term treatment group. The long-term treatment group had a higher incidence of atezolizumab-related thyroid dysfunction (31.9% vs. 10.7%, p < 0.001; median time to onset [mTTO], 2.8 months), dermatologic toxicity (29.0% vs. 14.7%, p=0.017; mTTO, 3.3 months), bevacizumab-related hypertension (44.9% vs. 22.0%, p=0.001; mTTO, 4.2 months), and proteinuria (69.6% vs. 38.4%, p < 0.001; mTTO, 6.8 months), compared to the short-term treatment group. Regarding liver function in the long-term treatment group, patients initially classified as Child-Pugh class A decreased from 87.0% to 75.4%, and albumin-bilirubin grade 1 decreased from 68.1% to 50.7% after 1 year of treatment.
Conclusion
The Ate/Bev long-term treatment group had a lower intrahepatic tumor burden, less portal vein tumor thrombosis, and better performance status and liver function at baseline. Atezolizumab-related immunological adverse events emerged relatively early in treatment compared to the bevacizumab-related. Additionally, some patients demonstrated liver function deterioration during long-term Ate/Bev treatment.
Keyword
Figure
Reference
-
References
1. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–90.
Article2. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018; 391:1163–73.
Article3. Llovet JM, Pinyol R, Kelley RK, El-Khoueiry A, Reeves HL, Wang XW, et al. Molecular pathogenesis and systemic therapies for hepatocellular carcinoma. Nat Cancer. 2022; 3:386–401.
Article4. Yang C, Zhang H, Zhang L, Zhu AX, Bernards R, Qin W, et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2023; 20:203–22.
Article5. Llovet JM, Hernandez-Gea V. Hepatocellular carcinoma: reasons for phase III failure and novel perspectives on trial design. Clin Cancer Res. 2014; 20:2072–9.
Article6. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021; 7:7.7. Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022; 19:151–72.
Article8. Sperandio RC, Pestana RC, Miyamura BV, Kaseb AO. Hepatocellular carcinoma immunotherapy. Annu Rev Med. 2022; 73:267–78.
Article9. Lee MS, Ryoo BY, Hsu CH, Numata K, Stein S, Verret W, et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020; 21:808–20.
Article10. Lee WS, Yang H, Chon HJ, Kim C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med. 2020; 52:1475–85.
Article11. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020; 382:1894–905.
Article12. NCCN clinical practice guidelines in oncology (NCCN Guidelines) for hepatocellular carcinoma, V.2.2023 [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network;2023. [cited 2023 Dec 31]. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1514.13. Kim C, Yang H, Kim I, Kang B, Kim H, Kim H, et al. Association of high levels of antidrug antibodies against atezolizumab with clinical outcomes and T-cell responses in patients with hepatocellular carcinoma. JAMA Oncol. 2022; 8:1825–9.
Article14. Yang H, Kang B, Ha Y, Lee SH, Kim I, Kim H, et al. High serum IL-6 correlates with reduced clinical benefit of atezolizumab and bevacizumab in unresectable hepatocellular carcinoma. JHEP Rep. 2023; 5:100672.
Article15. Zhu AX, Dayyani F, Yen CJ, Ren Z, Bai Y, Meng Z, et al. Alphafetoprotein as a potential surrogate biomarker for atezolizumab+bevacizumab treatment of hepatocellular carcinoma. Clin Cancer Res. 2022; 28:3537–45.16. Zhu AX, Abbas AR, de Galarreta MR, Guan Y, Lu S, Koeppen H, et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat Med. 2022; 28:1599–611.
Article17. Song YS, Yang H, Kang B, Cheon J, Kim I, Kim H, et al. Thyroid dysfunction after atezolizumab and bevacizumab is associated with favorable outcomes in hepatocellular carcinoma. Liver Cancer. 2024; 13:89–98.
Article18. An HJ, Chon HJ, Kim C. Peripheral blood-based biomarkers for immune checkpoint inhibitors. Int J Mol Sci. 2021; 22:9414.
Article19. Zheng Z, Zhou L, Gao S, Yang Z, Yao J, Zheng S. Prognostic role of C-reactive protein in hepatocellular carcinoma: a systematic review and meta-analysis. Int J Med Sci. 2013; 10:653–64.
Article20. Cheon J, Yoo C, Hong JY, Kim HS, Lee DW, Lee MA, et al. Efficacy and safety of atezolizumab plus bevacizumab in Korean patients with advanced hepatocellular carcinoma. Liver Int. 2022; 42:674–81.
Article21. Li DZ, Guo J, Song QK, Hu XJ, Bao XL, Lu J. Prognostic prediction of the platelet-to-lymphocyte ratio in hepatocellular carcinoma: a systematic review and meta-analysis. Transl Cancer Res. 2022; 11:4037–50.
Article22. Zhang D, Liu Z, Yin X, Qi X, Lu B, Liu Y, et al. Prognostic value of PIVKA-II in hepatocellular carcinoma patients receiving curative ablation: a systematic review and meta-analysis. Int J Biol Markers. 2018; 33:266–74.
Article23. Cheon J, Jung S, Kang B, Kim H, Kim C, Chon H. Organ-specific responses to atezolizumab plus bevacizumab in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2022; 40(16 Suppl):4076.
Article24. Kim HS, Kim CG, Hong JY, Kim IH, Kang B, Jung S, et al. The presence and size of intrahepatic tumors determine the therapeutic efficacy of nivolumab in advanced hepatocellular carcinoma. Ther Adv Med Oncol. 2022; 14:17588359221113266.
Article25. Yoest JM. Clinical features, predictive correlates, and pathophysiology of immune-related adverse events in immune checkpoint inhibitor treatments in cancer: a short review. Immunotargets Ther. 2017; 6:73–82.
Article26. Kudo M, Tsuchiya K, Shao YY, Finn RS, Galle PR, Ducreux M, et al. Impact of bevacizumab being skipped due to adverse events of special interest for bevacizumab in patients with unresectable hepatocellular carcinoma treated with atezolizumab plus bevacizumab: an exploratory analysis of the phase III IMbrave150 study. Liver Cancer. 2023; 13:401–12.
Article27. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022; 1:EVIDoa2100070.
Article28. Vogel A, Chan SL, Furuse J, Tak WY, Masi G, Varela M, et al. 79P outcomes by baseline liver function in patients with unresectable hepatocellular carcinoma treated with tremelimumab and durvalumab in the phase III Himalaya study. Ann Oncol. 2022; 33(Suppl 9):S1463–4.
Article29. Finn RS, Ryoo BY, Hsu CH, Li D, Burgoyne A, Cotter C, et al. Results from the MORPHEUS-liver study: phase Ib/II randomized evaluation of tiragolumab (tira) in combination with atezolizumab (atezo) and bevacizumab (bev) in patients with unresectable, locally advanced or metastatic hepatocellular carcinoma (uHCC). J Clin Oncol. 2023; 41(16 Suppl):4010.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Atezolizumab and bevacizumab for hepatocellular carcinoma: How to approach salvage therapy for non-responders?: Editorial on “Sorafenib vs. Lenvatinib in advanced hepatocellular carcinoma after atezolizumab/bevacizumab failure: A real-world study”
- Letter regarding “Feasibility of additional radiotherapy in patients with advanced hepatocellular carcinoma treated with atezolizumab plus bevacizumab”
- Concurrent transarterial radioembolization and combination atezolizumab/ bevacizumab treatment of infiltrative hepatocellular carcinoma with portal vein tumor thrombosis: a case report
- Favorable response of hepatocellular carcinoma with portal vein tumor thrombosis after radiotherapy combined with atezolizumab plus bevacizumab
- Clinical significance of the discrepancy between radiological findings and biochemical responses in atezolizumab plus bevacizumab for hepatocellular carcinoma