J Liver Cancer.
2023 Mar;23(1):225-229. 10.17998/jlc.2023.02.27.
Favorable response of hepatocellular carcinoma with portal vein tumor thrombosis after radiotherapy combined with atezolizumab plus bevacizumab
- Affiliations
-
- 1Department of Radiation Oncology, Yonsei Cancer Center, Heavy Ion Therapy Research Institute, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2540836
- DOI: http://doi.org/10.17998/jlc.2023.02.27
Abstract
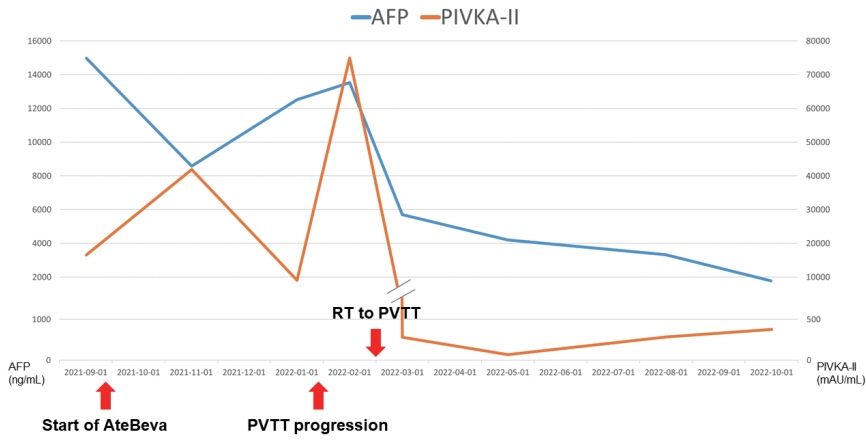
- Recently, the superiority of atezolizumab plus bevacizumab (AteBeva) over sorafenib was proven in the IMbrave150 trial, and AteBeva became the first-line systemic treatment for untreated, unresectable hepatocellular carcinoma (HCC). While the results are encouraging, more than half of patients with advanced HCC are still being treated in a palliative setting. Radiotherapy (RT) is known to induce immunogenic effects that may enhance the therapeutic efficacy of immune checkpoint inhibitors. Herein, we report the case of a patient with advanced HCC with massive portal vein tumor thrombosis treated with a combination of RT and AteBeva, who showed near complete response in tumor thrombosis and favorable response to HCC. Although this is a rare case, it shows the importance of reducing the tumor burden via RT to combination immunotherapy in patients with advanced HCC.
Figure
Reference
-
References
1. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017; 389:2492–2502.
Article2. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a nonrandomised, open-label phase 2 trial. Lancet Oncol. 2018; 19:940–952.3. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020; 382:1894–1905.
Article4. Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015; 16:e498–e509.
Article5. Bernstein MB, Krishnan S, Hodge JW, Chang JY. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol. 2016; 13:516–524.
Article6. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. IMbrave150: updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J Clin Oncol. 2021; 39 Suppl 3:267.
Article7. Cheon J, Yoo C, Hong JY, Kim HS, Lee DW, Lee MA, et al. Efficacy and safety of atezolizumab plus bevacizumab in Korean patients with advanced hepatocellular carcinoma. Liver Int. 2022; 42:674–681.
Article8. Rim CH, Yang DS, Park YJ, Yoon WS, Lee JA, Kim CY. Effectiveness of high-dose three-dimensional conformal radiotherapy in hepatocellular carcinoma with portal vein thrombosis. Jpn J Clin Oncol. 2012; 42:721–729.
Article9. Su F, Chen KH, Liang ZG, Wu CH, Li L, Qu S, et al. Comparison of three-dimensional conformal radiotherapy and hepatic resection in hepatocellular carcinoma with portal vein tumor thrombus. Cancer Med. 2018; 7:4387–4395.
Article10. Wei X, Jiang Y, Zhang X, Feng S, Zhou B, Ye X, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study. J Clin Oncol. 2019; 37:2141–2151.
Article11. Dall'Olio FG, Marabelle A, Caramella C, Garcia C, Aldea M, Chaput N, et al. Tumour burden and efficacy of immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2022; 19:75–90.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Durable complete response after discontinuation of atezolizumab-bevacizumab therapy in patients with hepatocellular carcinoma with portal vein tumor thrombosis: the first report
- Concurrent transarterial radioembolization and combination atezolizumab/ bevacizumab treatment of infiltrative hepatocellular carcinoma with portal vein tumor thrombosis: a case report
- Atezolizumab and bevacizumab for hepatocellular carcinoma: How to approach salvage therapy for non-responders?: Editorial on “Sorafenib vs. Lenvatinib in advanced hepatocellular carcinoma after atezolizumab/bevacizumab failure: A real-world study”
- A Case of Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis Treated by Hepatic Artery Injection Chemotherapy and Radiotherapy
- Management strategies for advanced hepatocellular carcinoma with portal vein tumor thrombosis