Cancer Res Treat.
2024 Oct;56(4):1219-1230. 10.4143/crt.2024.283.
Clinical and Radiologic Predictors of Response to Atezolizumab-Bevacizumab in Advanced Hepatocellular Carcinoma
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Gastroenterology, Liver Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2560255
- DOI: http://doi.org/10.4143/crt.2024.283
Abstract
- Purpose
This study aimed to identify clinical and radiologic characteristics that could predict response to atezolizumab-bevacizumab combination therapy in patients with advanced hepatocellular carcinoma (HCC).
Materials and Methods
This single-center retrospective study included 108 advanced HCC patients with intrahepatic lesions who were treated with atezolizumab-bevacizumab. Two radiologists independently analyzed imaging characteristics of the index tumor on pretreatment computed tomography. Predictive factors associated with progressive disease (PD) at the best response based on Response Evaluation Criteria in Solid Tumors, ver. 1.1 were evaluated using logistic regression analysis. Progression-free survival (PFS) was estimated by the Kaplan-Meier method and compared with the log-rank test.
Results
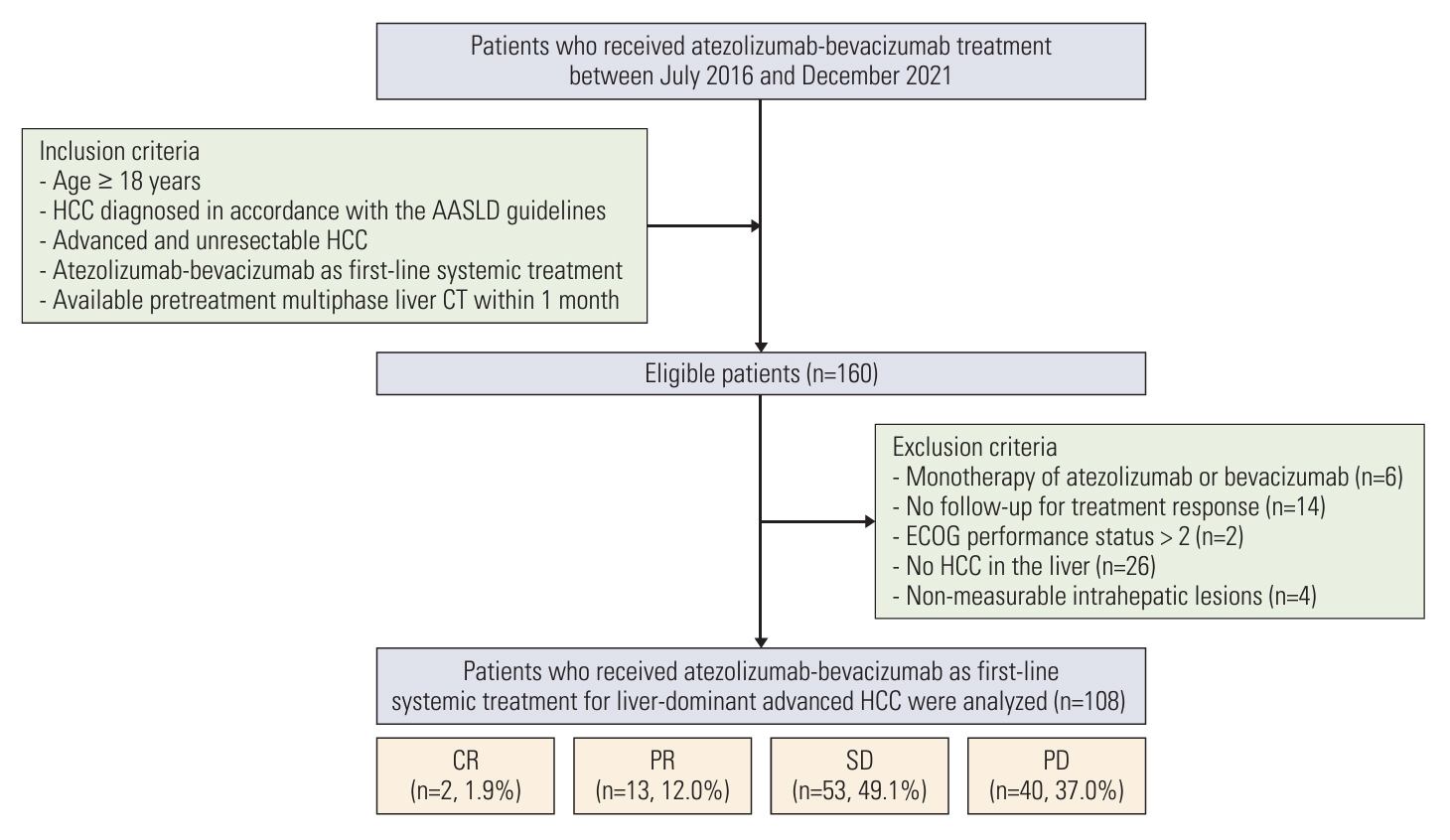
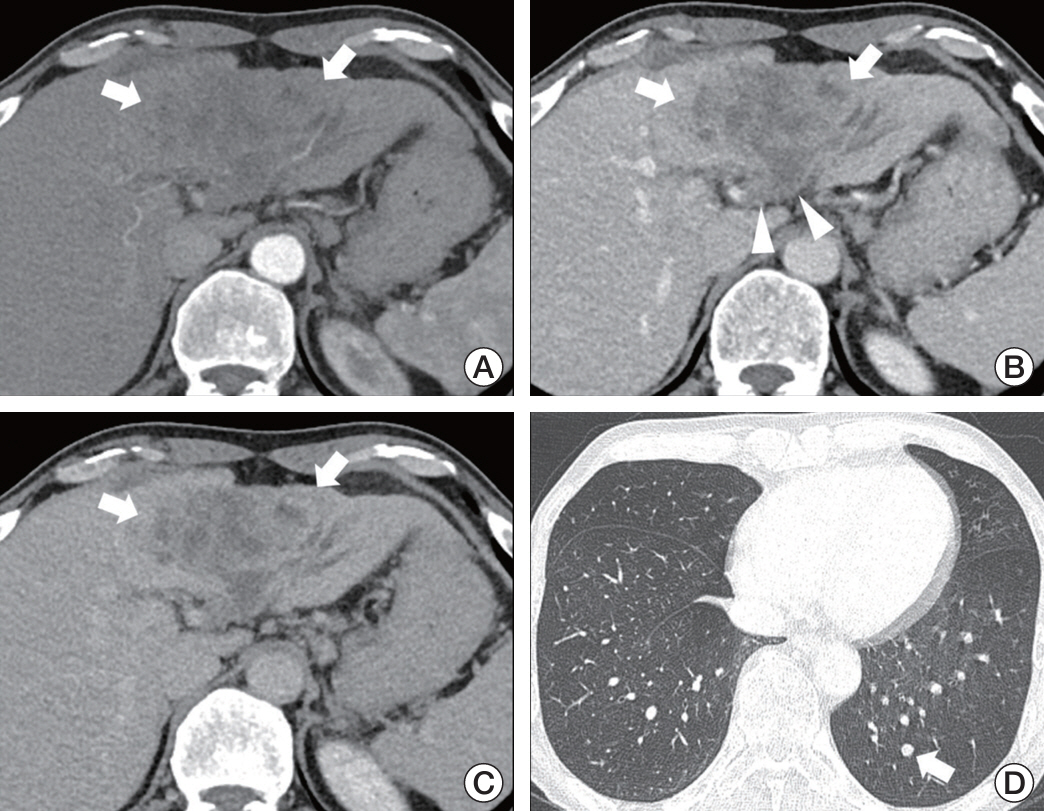
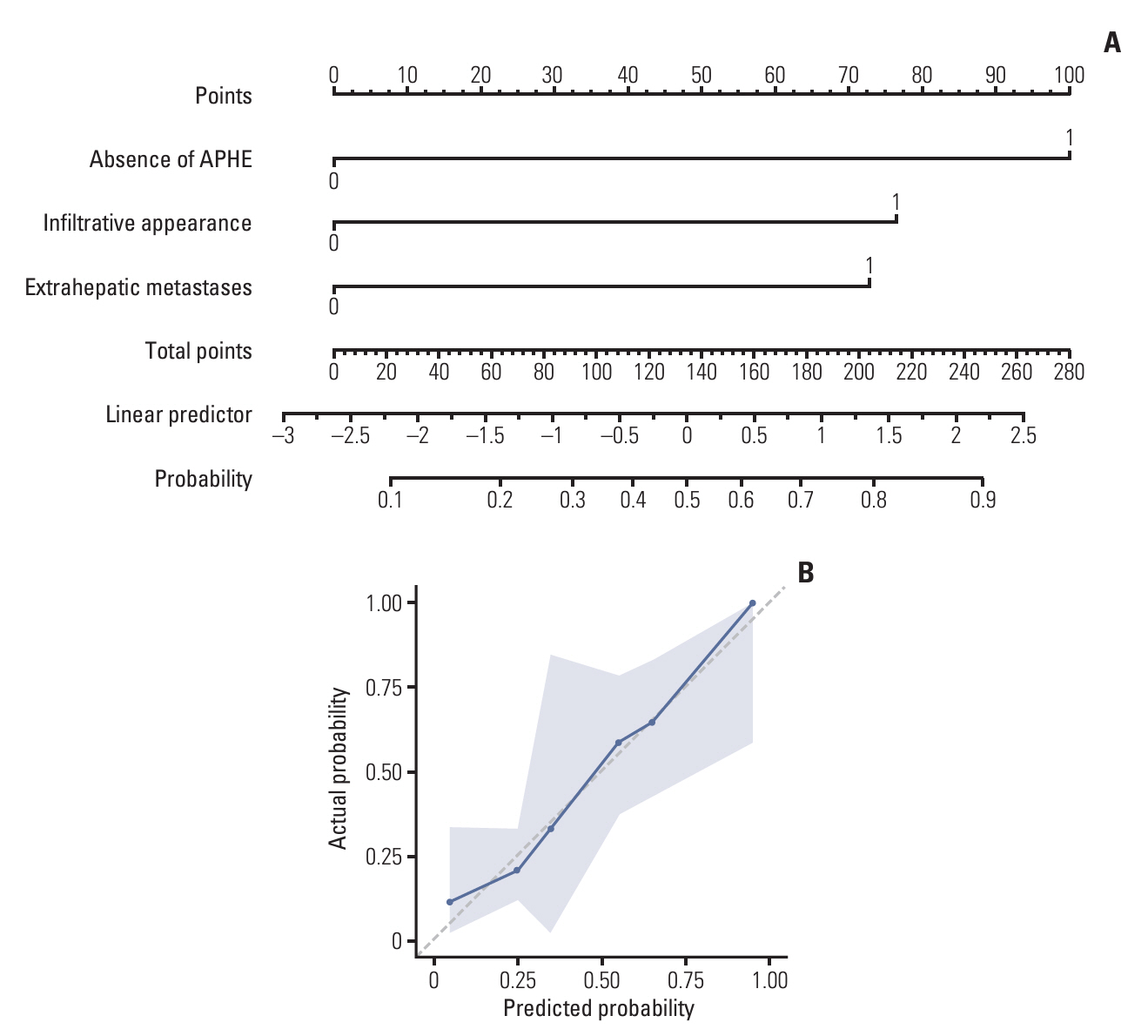
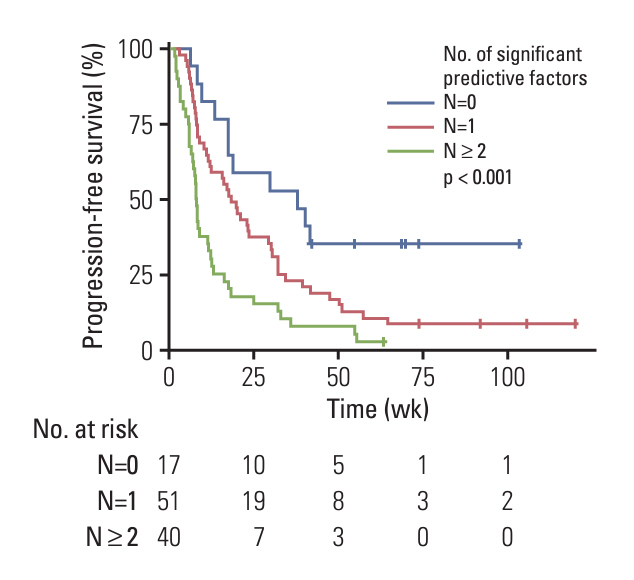
Of 108 patients with a median PFS of 15 weeks, 40 (37.0%) had PD during treatment. Factors associated with PD included the presence of extrahepatic metastases (adjusted odds ratio [aOR], 4.13; 95% confidence interval [CI], 1.19 to 14.35; p=0.03), the infiltrative appearance of the tumor (aOR, 3.07; 95% CI, 1.05 to 8.93; p=0.04), and the absence of arterial-phase hyperenhancement (APHE) (aOR, 6.34; 95% CI, 2.18 to 18.47; p < 0.001). Patients with two or more of these factors had a PD of 66.7% and a median PFS of 8 weeks, indicating a significantly worse outcome compared to the patients with one or no of these factors.
Conclusion
In patients with advanced HCC treated with atezolizumab-bevacizumab treatment, the absence of APHE, infiltrative appearance of the intrahepatic tumor, and presence of extrahepatic metastases were associated with poor response and survival. Evaluation of early response may be necessary in patients with these factors.
Keyword
Figure
Reference
-
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–49.
Article2. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018; 69:182–236.3. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018; 67:358–80.
Article4. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020; 382:1894–905.
Article5. Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019; 30:582–8.
Article6. Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, et al. Five-year overall survival for patients with advanced non‒small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol. 2019; 37:2518–27.
Article7. Brahmer JR, Govindan R, Anders RA, Antonia SJ, Sagorsky S, Davies MJ, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of non-small cell lung cancer (NSCLC). J Immunother Cancer. 2018; 6:75.
Article8. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016; 375:1823–33.
Article9. Sangro B, Sarobe P, Hervas-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021; 18:525–43.
Article10. Lu LC, Hsu C, Shao YY, Chao Y, Yen CJ, Shih IL, et al. Differential organ-specific tumor response to immune checkpoint inhibitors in hepatocellular carcinoma. Liver Cancer. 2019; 8:480–90.
Article11. Korean Liver Cancer Association; National Cancer Center Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol. 2022; 28:583–705.12. Fowler KJ, Burgoyne A, Fraum TJ, Hosseini M, Ichikawa S, Kim S, et al. Pathologic, molecular, and prognostic radiologic features of hepatocellular carcinoma. Radiographics. 2021; 41:1611–31.
Article13. CT/MRI LI-RADS v2018 CORE [Internet]. Reston, VA: American College of Radiology;2018. [cited 2024 May 1]. Available from: https://www.acr.org/-/media/ACR/Files/RADS/LIRADS/LI-RADS-2018-Core.pdf.14. LI-RADS lexicon table [Internet]. Reston, VA: American College of Radiology;2020. [cited 2024 May 1]. Available from: https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LIRADS-Lexicon-Table.pdf.15. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009; 10:25–34.
Article16. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018; 391:1163–73.
Article17. Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017; 389:56–66.
Article18. Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018; 379:54–63.
Article19. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017; 389:2492–502.
Article20. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018; 19:940–52.21. Osorio JC, Arbour KC, Le DT, Durham JN, Plodkowski AJ, Halpenny DF, et al. Lesion-level response dynamics to programmed cell death protein (PD-1) blockade. J Clin Oncol. 2019; 37:3546–55.
Article22. Yoneda N, Matsui O, Kobayashi S, Kitao A, Kozaka K, Inoue D, et al. Current status of imaging biomarkers predicting the biological nature of hepatocellular carcinoma. Jpn J Radiol. 2019; 37:191–208.
Article23. Brackenier C, Kinget L, Cappuyns S, Verslype C, Beuselinck B, Dekervel J. Unraveling the synergy between atezolizumab and bevacizumab for the treatment of hepatocellular carcinoma. Cancers (Basel). 2023; 15:348.
Article24. Hegde PS, Wallin JJ, Mancao C. Predictive markers of antiVEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol. 2018; 52:117–24.
Article25. Verbiest A, Renders I, Caruso S, Couchy G, Job S, Laenen A, et al. Clear-cell renal cell carcinoma: molecular characterization of IMDC Risk Groups and Sarcomatoid Tumors. Clin Genitourin Cancer. 2019; 17:e981–94.
Article26. Roussel E, Kinget L, Verbiest A, Boeckx B, Zucman-Rossi J, Couchy G, et al. Molecular underpinnings of glandular tropism in metastatic clear cell renal cell carcinoma: therapeutic implications. Acta Oncol. 2021; 60:1499–506.
Article27. Kuwano A, Yada M, Miyazaki Y, Tanaka K, Koga Y, Ohishi Y, et al. An imaging feature predicts efficacy of atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. Cancer Diagn Progn. 2023; 3:468–74.
Article28. Katyal S, Oliver JH 3rd, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000; 216:698–703.
Article29. Choi WM, Lee D, Shim JH, Kim KM, Lim YS, Lee HC, et al. Effectiveness and safety of nivolumab in Child-Pugh B patients with hepatocellular carcinoma: a real-world cohort study. Cancers (Basel). 2020; 12:1968.
Article30. Yopp AC, Mokdad A, Zhu H, Mansour JC, Balch GC, Choti MA, et al. Infiltrative hepatocellular carcinoma: natural history and comparison with multifocal, nodular hepatocellular carcinoma. Ann Surg Oncol. 2015; 22 Suppl 3:S1075–82.
Article31. Awiwi MO, Elsayes KM, Mohamed YI, Altameemi L, Gjoni M, Irshad OM, et al. The prognostic value of baseline clinical and radiologic imaging features in patients with unresectable hepatocellular carcinoma treated with atezolizumab plus bevacizumab. J Hepatocell Carcinoma. 2022; 9:913–27.
Article32. Mansour D, McPherson S. Management of decompensated cirrhosis. Clin Med (Lond). 2018; 18:s60–5.
Article33. Hung TH, Tseng CW, Tsai CC, Tsai CC, Tseng KC, Hsieh YH. The long-term outcomes of cirrhotic patients with pleural effusion. Saudi J Gastroenterol. 2018; 24:46–51.
Article34. Lee JH, Lee JM, Kim SJ, Baek JH, Yun SH, Kim KW, et al. Enhancement patterns of hepatocellular carcinomas on multiphasicmultidetector row CT: comparison with pathological differentiation. Br J Radiol. 2012; 85:e573–83.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Atezolizumab and bevacizumab for hepatocellular carcinoma: How to approach salvage therapy for non-responders?: Editorial on “Sorafenib vs. Lenvatinib in advanced hepatocellular carcinoma after atezolizumab/bevacizumab failure: A real-world study”
- Favorable response of hepatocellular carcinoma with portal vein tumor thrombosis after radiotherapy combined with atezolizumab plus bevacizumab
- Concurrent transarterial radioembolization and combination atezolizumab/ bevacizumab treatment of infiltrative hepatocellular carcinoma with portal vein tumor thrombosis: a case report
- Clinical significance of the discrepancy between radiological findings and biochemical responses in atezolizumab plus bevacizumab for hepatocellular carcinoma
- Complete response in hepatocellular carcinoma with lymph node metastasis by combination therapy of atezolizumab and bevacizumab: a case report