Cancer Res Treat.
2024 Oct;56(4):1096-1104. 10.4143/crt.2024.092.
A Nationwide Study on HER2-Low Breast Cancer in South Korea: Its Incidence of 2022 Real World Data and the Importance of Immunohistochemical Staining Protocols
- Affiliations
-
- 1Department of Pathology, Yeungnam University College of Medicine, Daegu, Korea
- 2Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Department of Pathology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 4Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 5Department of Pathology, Chonnam National University Medical School, Gwangju, Korea
- 6Department of Pathology, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Yangsan, Korea
- 7Department of Pathology, Chungbuk National University College of Medicine, Cheongju, Korea
- 8Department of Pathology, Saegyaero Hospital, Busan, Korea
- 9Department of Pathology, Chung-Ang University College of Medicine, Seoul, Korea
- 10Department of Pathology, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
- 11Department of Pathology, Konkuk University School of Medicine, Seoul, Korea
- 12Department of Pathology, Good Gang-an Hospital, Busan, Korea
- 13Department of Pathology, Jeju National University School of Medicine, Jeju, Korea
- 14Department of Pathology, Wonkwang University Hospital, Wonkwang University School of Medicine, Iksan, Korea
- 15Department of Pathology, Keimyung University School of Medicine, Daegu, Korea
- 16Department of Pathology, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- 17Department of Pathology, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Incheon, Korea
- 18Department of Pathology, Seoul Clinical Laboratories, Yongin, Korea
- 19Department of Pathology, Ulsan University Hospital, Ulsan University, College of Medicine, Ulsan, Korea
- 20Department of Pathology, Chungnam National University Sejong Hospital, Sejong, Korea
- 21Department of Pathology, Jeonbuk National University Medical School, Jeonju, Korea
- 22Department of Pathology, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea
- 23Department of Pathology, Nowon Eulji University Hospital, Seoul, Korea
- 24Department of Pathology, Pusan National University Hospital, Busan, Korea
- 25Department of Pathology, CHA Gangnam Medical Center, CHA University School of Medicine, Seoul, Korea
- KMID: 2560244
- DOI: http://doi.org/10.4143/crt.2024.092
Abstract
- Purpose
Notable effectiveness of trastuzumab deruxtecan in patients with human epidermal growth factor receptor 2 (HER2)–low advanced breast cancer (BC) has focused pathologists’ attention. We studied the incidence and clinicopathologic characteristics of HER2-low BC, and the effects of immunohistochemistry (IHC) associated factors on HER2 IHC results.
Materials and Methods
The Breast Pathology Study Group of the Korean Society of Pathologists conducted a nationwide study using real-world data on HER2 status generated between January 2022 and December 2022. Information on HER2 IHC protocols at each participating institution was also collected.
Results
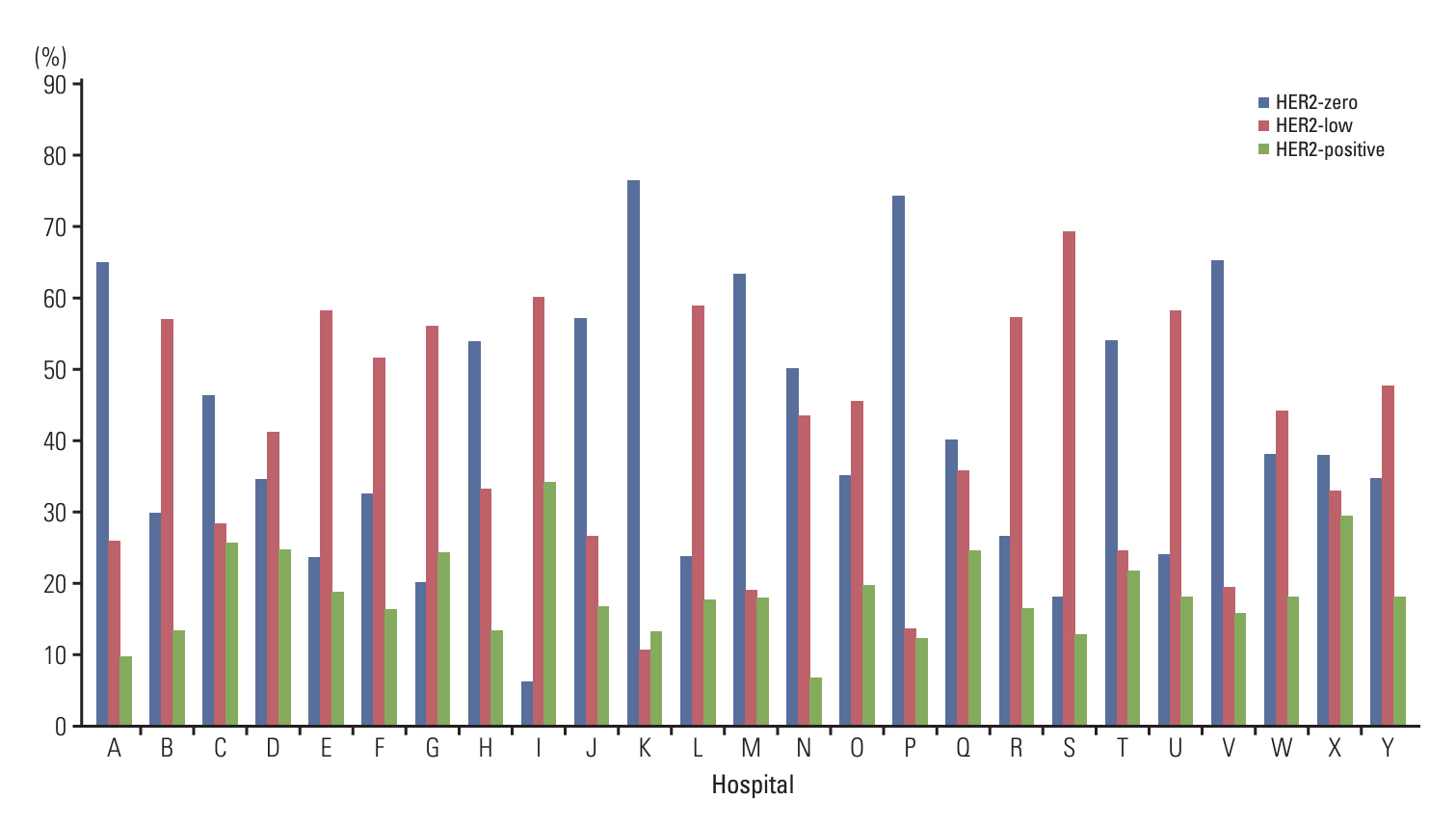
Total 11,416 patients from 25 institutions included in this study. Of these patients, 40.7% (range, 6.0% to 76.3%) were classified as HER2-zero, 41.7% (range, 10.5% to 69.1%) as HER2-low, and 17.5% (range, 6.7% to 34.0%) as HER2-positive. HER2-low tumors were associated with positive estrogen receptor and progesterone receptor statuses (p < 0.001 and p < 0.001, respectively). Antigen retrieval times (≥ 36 minutes vs. < 36 minutes) and antibody incubation times (≥ 12 minutes vs. < 12 minutes) affected on the frequency of HER2 IHC 1+ BC at institutions using the PATHWAY HER2 (4B5) IHC assay and BenchMark XT or Ultra staining instruments. Furthermore, discordant results between core needle biopsy and subsequent resection specimen HER2 statuses were observed in 24.1% (787/3,259) of the patients.
Conclusion
The overall incidence of HER2-low BC in South Korea concurs with those reported in previously published studies. Significant inter-institutional differences in HER2 IHC protocols were observed, and it may have impact on HER2-low status. Thus, we recommend standardizing HER2 IHC conditions to ensure precise patient selection for targeted therapy.
Keyword
Figure
Reference
-
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–49.
Article2. Kim H, Lee SB, Kim J, Chung IY, Kim HJ, Ko BS, et al. Improvement of survival in Korean breast cancer patients over a 14-year period: a large-scale single-center study. PLoS One. 2022; 17:e0265533.
Article3. Wolff AC, Hammond ME, Allison KH, Harvey BE, Mangu PB, Bartlett JM, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol. 2018; 36:2105–22.
Article4. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007; 131:18–43.
Article5. Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013; 31:3997–4013.
Article6. Tozbikian G, Krishnamurthy S, Bui MM, Feldman M, Hicks DG, Jaffer S, et al. Emerging landscape of targeted therapy of breast cancers with low human epidermal growth factor receptor 2 protein expression. Arch Pathol Lab Med. 2024; 148:242–55.
Article7. Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022; 387:9–20.
Article8. Tarantino P, Hamilton E, Tolaney SM, Cortes J, Morganti S, Ferraro E, et al. HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol. 2020; 38:1951–62.
Article9. Gampenrieder SP, Rinnerthaler G, Tinchon C, Petzer A, Balic M, Heibl S, et al. Landscape of HER2-low metastatic breast cancer (MBC): results from the Austrian AGMT_MBC-Registry. Breast Cancer Res. 2021; 23:112.
Article10. Horisawa N, Adachi Y, Takatsuka D, Nozawa K, Endo Y, Ozaki Y, et al. The frequency of low HER2 expression in breast cancer and a comparison of prognosis between patients with HER2-low and HER2-negative breast cancer by HR status. Breast Cancer. 2022; 29:234–41.
Article11. Zhang G, Ren C, Li C, Wang Y, Chen B, Wen L, et al. Distinct clinical and somatic mutational features of breast tumors with high-, low-, or non-expressing human epidermal growth factor receptor 2 status. BMC Med. 2022; 20:142.
Article12. Baez-Navarro X, van Bockstal MR, Andrinopoulou ER, van Deurzen CH. HER2-low breast cancer: incidence, clinicopathologic features, and survival outcomes from real-world data of a large nationwide cohort. Mod Pathol. 2023; 36:100087.
Article13. Bergeron A, Bertaut A, Beltjens F, Charon-Barra C, Amet A, Jankowski C, et al. Anticipating changes in the HER2 status of breast tumours with disease progression-towards better treatment decisions in the new era of HER2-low breast cancers. Br J Cancer. 2023; 129:122–34.
Article14. Schettini F, Chic N, Braso-Maristany F, Pare L, Pascual T, Conte B, et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. 2021; 7:1.
Article15. Yang L, Liu Y, Han D, Fu S, Guo S, Bao L, et al. Clinical genetic features and neoadjuvant chemotherapy response in HER2-low breast cancers: a retrospective, multicenter cohort study. Ann Surg Oncol. 2023; 30:5653–62.
Article16. Tarantino P, Gandini S, Nicolo E, Trillo P, Giugliano F, Zagami P, et al. Evolution of low HER2 expression between early and advanced-stage breast cancer. Eur J Cancer. 2022; 163:35–43.
Article17. Allison KH, Hammond ME, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, et al. Estrogen and progesterone receptor testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists guideline update. Arch Pathol Lab Med. 2020; 144:545–63.
Article18. Ruschoff J, Lebeau A, Kreipe H, Sinn P, Gerharz CD, Koch W, et al. Assessing HER2 testing quality in breast cancer: variables that influence HER2 positivity rate from a large, multicenter, observational study in Germany. Mod Pathol. 2017; 30:217–26.
Article19. Wolff AC, Somerfield MR, Dowsett M, Hammond ME, Hayes DF, McShane LM, et al. Human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2023; 147:993–1000.
Article20. Miglietta F, Griguolo G, Bottosso M, Giarratano T, Lo Mele M, Fassan M, et al. Evolution of HER2-low expression from primary to recurrent breast cancer. NPJ Breast Cancer. 2021; 7:137.
Article21. Al Haddabi I, Qureshi A, Saparamadu A, Al Hamdani A, Al Riyami M, Ganguly S. Inter-observer agreement in reporting HER 2 Neu protein over expression by immunohistochemistry. Indian J Pathol Microbiol. 2014; 57:201–4.
Article22. Md Pauzi SH, Masir N, Yahaya A, Mohammed F, Tizen Laim NM, Mustangin M, et al. HER2 testing by immunohistochemistry in breast cancer: a multicenter proficiency ring study. Indian J Pathol Microbiol. 2021; 64:677–82.23. Karakas C, Tyburski H, Turner BM, Wang X, Schiffhauer LM, Katerji H, et al. Interobserver and interantibody reproducibility of HER2 immunohistochemical scoring in an enriched HER2-low-expressing breast cancer cohort. Am J Clin Pathol. 2023; 159:484–91.
Article24. Layfield LJ, Frazier S, Esebua M, Schmidt RL. Interobserver reproducibility for HER2/neu immunohistochemistry: a comparison of reproducibility for the HercepTest and the 4B5 antibody clone. Pathol Res Pract. 2016; 212:190–5.
Article25. Hicks DG, Schiffhauer L. Standardized assessment of the HER2 status in breast cancer by immunohistochemistry. Lab Med. 2011; 42:459–67.
Article26. Krenacs L, Krenacs T, Stelkovics E, Raffeld M. Heat-induced antigen retrieval for immunohistochemical reactions in routinely processed paraffin sections. Methods Mol Biol. 2010; 588:103–19.
Article27. Boenisch T. Heat-induced antigen retrieval restores electrostatic forces: prolonging the antibody incubation as an alternative. Appl Immunohistochem Mol Morphol. 2002; 10:363–7.
Article28. Ramos-Vara JA, Miller MA. When tissue antigens and antibodies get along: revisiting the technical aspects of immunohistochemistry--the red, brown, and blue technique. Vet Pathol. 2014; 51:42–87.29. Chen YY, Yang CF, Hsu CY. The impact of modified staining method on HER2 immunohistochemical staining for HER2-low breast cancer. Pathology. 2024; 56:122–4.
Article30. Garrido C, Manoogian M, Ghambire D, Lucas S, Karnoub M, Olson MT, et al. Analytical and clinical validation of PATHWAY Anti-HER-2/neu (4B5) antibody to assess HER2-low status for trastuzumab deruxtecan treatment in breast cancer. Virchows Arch. 2024; 484:1005–14.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis and Treatment of HER2-Positive Breast Cancer
- Exploring the clinicopathological parameters of HER2 low breast cancers: insights from a retrospective cohort study
- Differences in HER2-0 and HER2-low Breast Cancer: Androgen Receptor and Programmed Death Ligand 1 as Predictive Factors
- HER2-Low Breast Cancer: Now and in the Future
- Real-World Evidence of Trastuzumab, Pertuzumab, and Docetaxel Combination as a First-Line Treatment for Korean Patients with HER2-Positive Metastatic Breast Cancer