Anat Cell Biol.
2024 Sep;57(3):446-458. 10.5115/acb.24.054.
Edible mushroom (Pleurotus cornucopiae) extract vs. glibenclamide on alloxan induced diabetes: sub-acute in vivo study of Nrf2 expression and renal toxicity
- Affiliations
-
- 1Department of Anatomy, Faculty of Basic Medical Sciences, College of Medical Sciences, Alex Ekwueme Federal University Ndufu-Alike, Ebonyi State, Abakaliki, Nigeria
- 2Department of Physiology, Faculty of Basic Medical Sciences, College of Medical Sciences, Alex Ekwueme Federal University Ndufu-Alike, Ebonyi State, Abakaliki, Nigeria
- 3Department of Histology and Embryology, School of Anatomical Sciences, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
- KMID: 2559764
- DOI: http://doi.org/10.5115/acb.24.054
Abstract
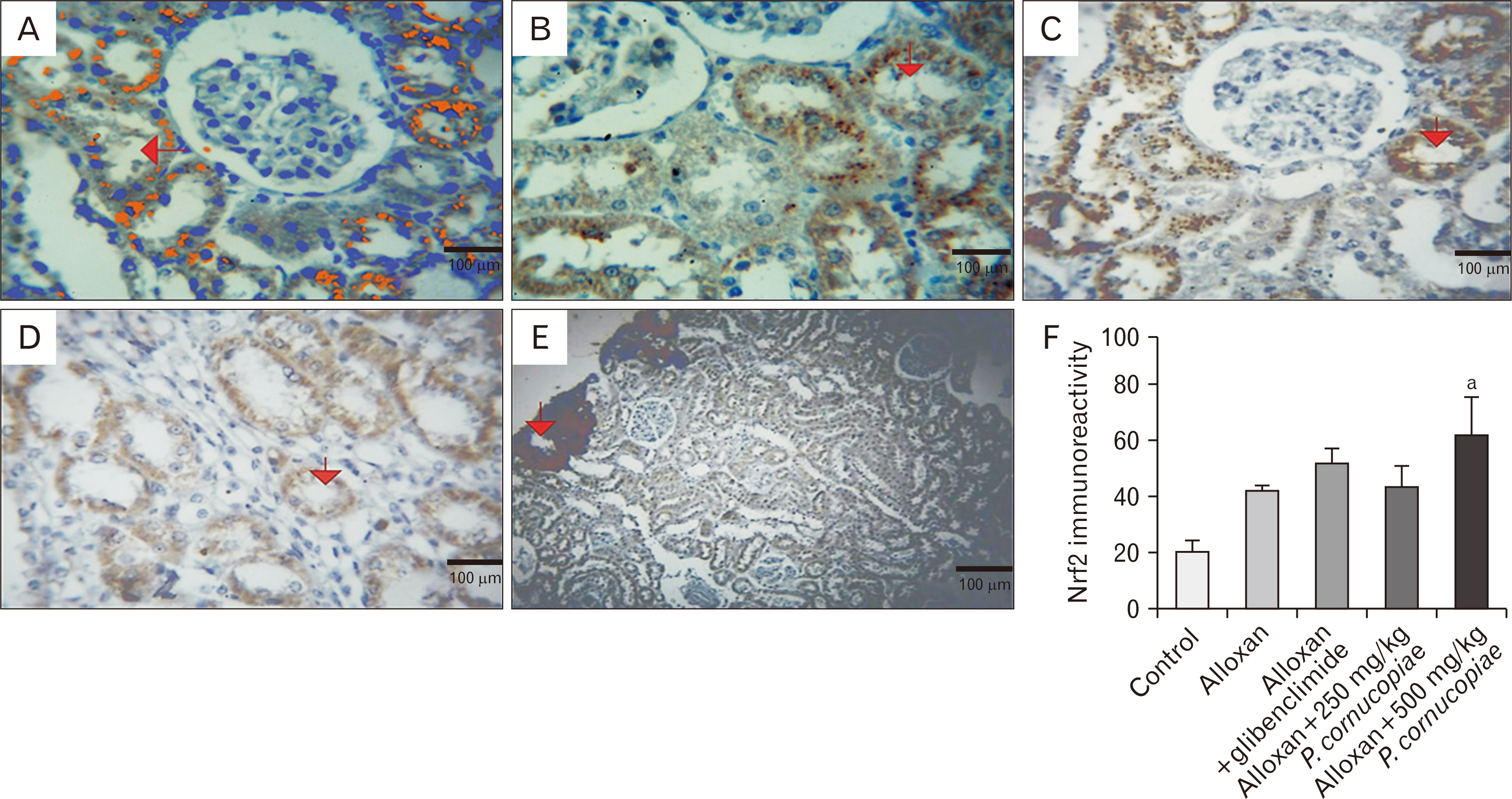
- The study aims to compare the action of Pleurotus cornucopiae and glibenclamide on alloxan-induced diabetes and ascertain how an aqueous extract of the edible mushroom regulates the expression of nuclear factor erythroid 2-related factor 2 (Nrf2), oxidative stress biomarkers and renal toxicity in a diabetic male Wistar rat model. Twenty-five adult male Wistar rats were randomly grouped into five groups with five rats per. Group 1 and those in the treatment groups received normal feed and water ad libitum. Group 2 received intraperitoneal administration of alloxan monohydrate (150 mg/kg body weight). Group 3 received alloxan monohydrate and glibenclamide (5 mg/kg body weight bwt), group 4 received alloxan monohydrate plus the extract (250 mg/kg bwt) and group 5 received alloxan monohydrate plus the extract (500 mg/kg bwt). The administration of glibenclamide plus the extract was oral for 14 days. Glibenclamide and the extract lowered blood glucose level, catalase, and glutathione peroxidase activities, increased the superoxide dismutase (SOD) activity in rats with alloxan induced diabetes. The extract at 500 mg/kg bwt reduced the plasma urea and sodium concentration in the treated rats. The extract and glibenclamide could detoxify alloxan and restore its induced renal degeneration and glomeruli atrophy, intra renal hemorrhage and inflammation and oxidative biomarkers through activation of Nrf2 expression. The drug glibenclamide and P. cornucopiae have appreciable hypoglycemic activity and potential to restore the normal renal architecture in the rats, hence they offer similar curative effects. Additionally, the extract at 500 mg/kg bwt activated SOD and Nrf2 expression more than glibenclamide in rats with alloxan-induced diabetes.
Keyword
Figure
Reference
-
References
1. Sicree R, Shaw J, Zimmet P. Gan D, editor. The global burden. Diabetes and impaired glucose tolerance. Prevalence and projections. Diabetes Atlas. 3rd ed. International Diabetes Federation;2006. p. 16–103.2. Owembabazi E, Nkomozepi P, Calvey T, Mbajiorgu EF. 2023; Co-administration of alcohol and combination antiretroviral therapy (cART) in male Sprague Dawley rats: a study on testicular morphology, oxidative and cytokines perturbations. Anat Cell Biol. 56:236–51. DOI: 10.5115/acb.22.229. PMID: 36759974. PMCID: PMC10319480.3. Collins AJ, Kasiske B, Herzog C, Chen SC, Everson S, Constantini E, Grimm R, McBean M, Xue J, Chavers B, Matas A, Manning W, Louis T, Pan W, Liu J, Li S, Roberts T, Dalleska F, Snyder J, Ebben J, Frazier E, Sheets D, Johnson R, Li S, Dunning S, Berrini D, Guo H, Solid C, Arko C, Daniels F, Wang X, Forrest B, Gilbertson D, St Peter W, Frederick P, Eggers P, Agodoa L. 2003; Excerpts from the United States Renal Data System 2003 annual data report: atlas of end-stage renal disease in the United States. Am J Kidney Dis. 42(6 Suppl 5):A5–7. S1–230. DOI: 10.1053/j.ajkd.2004.10.009. PMID: 14655179.4. Church DS, Barker P, Burling KA, Shinwari SK, Kennedy C, Smith D, Macfarlane DP, Kernohan A, Stears A, Karamat MA, Whyte K, Narendran P, Halsall DJ, Semple RK. 2023; Diagnosis and treatment of anti-insulin antibody-mediated labile glycaemia in insulin-treated diabetes. Diabet Med. 40:e15194. DOI: 10.1111/dme.15194. PMID: 37562398. PMCID: PMC10946589.5. Yenet A, Nibret G, Tegegne BA. 2023; Challenges to the availability and affordability of essential medicines in African countries: a scoping review. Clinicoecon Outcomes Res. 15:443–58. DOI: 10.2147/CEOR.S413546. PMID: 37332489. PMCID: PMC10276598.6. Ugwu E, Young E, Nkpozi M. 2020; Diabetes care knowledge and practice among primary care physicians in Southeast Nigeria: a cross-sectional study. BMC Fam Pract. 21:128. DOI: 10.1186/s12875-020-01202-0. PMID: 32611395. PMCID: PMC7330977.7. Ogbera AO, Ekpebegh C. 2014; Diabetes mellitus in Nigeria: the past, present and future. World J Diabetes. 5:905–11. DOI: 10.4239/wjd.v5.i6.905. PMID: 25512795. PMCID: PMC4265879.8. Uloko AE, Musa BM, Ramalan MA, Gezawa ID, Puepet FH, Uloko AT, Borodo MM, Sada KB. 2018; Prevalence and risk factors for diabetes mellitus in Nigeria: a systematic review and meta-analysis. Diabetes Ther. 9:1307–16. DOI: 10.1007/s13300-018-0441-1. PMID: 29761289. PMCID: PMC5984944.9. Okolo KO, Siminialayi IM, Orisakwe OE. 2016; Protective effects of Pleurotus tuber-regium on carbon- tetrachloride induced testicular injury in Sprague Dawley rats. Front Pharmacol. 7:480. DOI: 10.3389/fphar.2016.00480. PMID: 28018218. PMCID: PMC5156682.10. Golak-Siwulska I, Kałużewicz A, Spiżewski T, Siwulski M, Sobieralski K. 2018; Bioactive compounds and medicinal properties of Oyster mushrooms (Pleurotus sp.). Folia Hortic. 30:191–201. DOI: 10.2478/fhort-2018-0012.11. Izham I, Avin F, Raseetha S. 2022; Systematic review: heat treatments on phenolic content, antioxidant activity, and sensory quality of Malaysian mushroom: oyster (Pleurotus spp.) and black jelly (Auricularia spp.). Front Sustain Food Syst. 6:882939. DOI: 10.3389/fsufs.2022.882939.12. Khan MA, Tania M. 2012; Nutritional and medicinal importance of Pleurotus mushrooms: an overview. Food Rev Int. 28:313–29. DOI: 10.1080/87559129.2011.637267.13. Joshi M, Sagar A. 2014; In vitro free radical scavenging activity of a wild edible mushroom, Sparassis crispa (Wulf.) Fr., from North Western Himalayas, India. J Mycol. 2014:748531. DOI: 10.1155/2014/748531.14. Wasser SP. 2014; Medicinal mushroom science: current perspectives, advances, evidences, and challenges. Biomed J. 37:345–56. DOI: 10.4103/2319-4170.138318. PMID: 25179726.15. Lindequist U, Niedermeyer TH, Jülich WD. 2005; The pharmacological potential of mushrooms. Evid Based Complement Alternat Med. 2:285–99. DOI: 10.1093/ecam/neh107. PMID: 16136207. PMCID: PMC1193547.16. Guillamón E, García-Lafuente A, Lozano M, D'Arrigo M, Rostagno MA, Villares A, Martínez JA. 2010; Edible mushrooms: role in the prevention of cardiovascular diseases. Fitoterapia. 81:715–23. DOI: 10.1016/j.fitote.2010.06.005. PMID: 20550954.17. Farquhar WB, Edwards DG, Jurkovitz CT, Weintraub WS. 2015; Dietary sodium and health: more than just blood pressure. J Am Coll Cardiol. 65:1042–50. DOI: 10.1016/j.jacc.2014.12.039. PMID: 25766952. PMCID: PMC5098396.18. Elsayed EA, El Enshasy H, Wadaan MA, Aziz R. 2014; Mushrooms: a potential natural source of anti-inflammatory compounds for medical applications. Mediators Inflamm. 2014:805841. DOI: 10.1155/2014/805841. PMID: 25505823. PMCID: PMC4258329.19. Okolo KO, Orisakwe OE. 2020; In vitro antioxidants and hepatoprotective effects of Pleurotus tuber-regium on carbon tetrachloride-treated rats. J Basic Clin Physiol Pharmacol. 32:67–78. DOI: 10.1515/jbcpp-2020-0034. PMID: 32833668.20. Rajasekaran S, Sivagnanam K, Subramanian S. 2005; Antioxidant effect of Aloe vera gel extract in streptozotocin-induced diabetes in rats. Pharmacol Rep. 57:90–6. DOI: 10.1211/0022357055416. PMID: 15849382.21. Gray AM, Flatt PR. 1998; Insulin-releasing and insulin-like activity of Agaricus campestris (mushroom). J Endocrinol. 157:259–66. DOI: 10.1677/joe.0.1570259. PMID: 9659289.22. Okigbo RN, Ezebo RO, Nwatu CM, Omumuabuike JN, Esimai GB. 2021; A study on cultivation of indigenous mushrooms in South Eastern Nigeria. World News Nat Sci. 34:154–64.23. Wang H, Gao J, Ng TB. 2000; A new lectin with highly potent antihepatoma and antisarcoma activities from the oyster mushroom Pleurotus ostreatus. Biochem Biophys Res Commun. 275:810–6. DOI: 10.1006/bbrc.2000.3373. PMID: 10973803.24. Aloke C, Emelike CU, Obasi NA, Ogbu PN, Edeogu CU, Uzomba GC, Ekakitie O, Iyaniwura AA, Okoro CC, Okey BP, Aninjoku GG, Ushahemba BC. 2021; HPLC profiling and studies on Copaifera salikounda methanol leaf extract on phenylhydrazine-induced hematotoxicity and oxidative stress in rats. Arabian J Chem. 14:103428. DOI: 10.1016/j.arabjc.2021.103428.25. Harborne JB. Phytochemical methods. 3rd ed. Chapman and Hall Ltd;1993. p. 49–188.26. Adebiyi AO. 2018; Distribution of phytochemicals and some anti-nutrients in selected edible mushrooms in Ekiti State, Nigeria. Int J Sci Eng Invent. 2018:10–3. DOI: 10.23958/10.23958/ijsei/vol04-i09/88.27. Wong F, Chai TT, Tan SL, Yong AL. 2013; Evaluation of bioactivities and phenolic content of selected edible mushrooms in Malaysia. Trop J Pharm Res. 12:1011–6. DOI: 10.4314/tjpr.v12i6.21.28. Yılmaz A, Yıldız S, Kılıç C, Can Z. 2017; Total phenolics, flavonoids, tannin contents and antioxidant properties of Pleurotus ostreatus cultivated on different wastes and sawdust. Int J Sec Metab. 4:1–9. DOI: 10.21448/ijsm.252052.29. Gul R, Jan SU, Faridullah S, Sherani S, Jahan N. 2017; Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from Ephedra intermedia indigenous to Balochistan. ScientificWorldJournal. 2017:5873648. DOI: 10.1155/2017/5873648. PMID: 28386582. PMCID: PMC5366796.30. National Research Council. Guide for the care and use of laboratory animals. 6th ed. National Institutes of Health;1985. p. 85–123.31. Balsells M, García-Patterson A, Solà I, Roqué M, Gich I, Corcoy R. 2015; Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 350:h102. DOI: 10.1136/bmj.h102. PMID: 25609400. PMCID: PMC4301599.32. Njogu SM, Arika WM, Machocho AK, Ngeranwa JJN, Njagi ENM. 2018; In vivo hypoglycemic effect of Kigelia africana (Lam): studies with alloxan-induced diabetic mice. J Evid Based Integr Med. 23:2515690X18768727. DOI: 10.1177/2515690X18768727. PMID: 29651878. PMCID: PMC5901018.33. Lenzen S. 2008; The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 51:216–26. DOI: 10.1007/s00125-007-0886-7. PMID: 18087688.34. Singh R, Gholipourmalekabadi M, Shafikhani SH. 2024; Animal models for type 1 and type 2 diabetes: advantages and limitations. Front Endocrinol (Lausanne). 15:1359685. DOI: 10.3389/fendo.2024.1359685. PMID: 38444587. PMCID: PMC10912558.35. Rahman SS, Yasmin N, Rahman ATMM, Zaman A, Rahman MH, Rouf SMA. 2017; Evaluation and optimization of effective-dose of alloxan for inducing type-2 diabetes mellitus in Long Evans rat. Indian J Pharm Educ Res. 51(4 Suppl):S661–6. DOI: 10.5530/ijper.51.4s.96.36. Devaki K, Beulah U, Akila G, Narmadha R, Gopalakrishnan VK. 2011; Glucose lowering effect of aqueous extract of Bauhinia tomentosa L. on alloxan induced type 2 diabetes mellitus in wistar albino rats. J Basic Clin Pharm. 2:167–74. PMID: 24826019. PMCID: PMC3979230.37. Maroof K, Gan SH. Boyacioglu D, editor. Bee products and diabetes mellitus. Bee Products and Their Applications in the Food and Pharmaceutical Industries. Academic Press;2022. p. 63–114. DOI: 10.1016/B978-0-323-85400-9.00012-5.38. Fajarwati I, Solihin DD, Wresdiyati T, Batubara I. 2023; Self-recovery in diabetic Sprague Dawley rats induced by intraperitoneal alloxan and streptozotocin. Heliyon. 9:e15533. DOI: 10.1016/j.heliyon.2023.e15533. PMID: 37159693. PMCID: PMC10163600.39. Kottaisamy CPD, Raj DS, Prasanth Kumar V, Sankaran U. 2021; Experimental animal models for diabetes and its related complications-a review. Lab Anim Res. 37:23. DOI: 10.1186/s42826-021-00101-4. PMID: 34429169. PMCID: PMC8385906.40. Ighodaro OM, Adeosun AM, Akinloye OA. 2017; Alloxan-induced diabetes, a common model for evaluating the glycemic-control potential of therapeutic compounds and plants extracts in experimental studies. Medicina (Kaunas). 53:365–74. DOI: 10.1016/j.medici.2018.02.001. PMID: 29548636.41. Sinha AK. 1972; Colorimetric assay of catalase. Anal Biochem. 47:389–94. DOI: 10.1016/0003-2697(72)90132-7. PMID: 4556490.42. Fridovich I. 1989; Superoxide dismutases. An adaptation to a paramagnetic gas. J Biol Chem. 264:7761–4. DOI: 10.1016/S0021-9258(18)83102-7. PMID: 2542241.43. Buege JA, Aust SD. 1978; Microsomal lipid peroxidation. Methods Enzymol. 52:302–10. DOI: 10.1016/S0076-6879(78)52032-6. PMID: 672633.44. Ubhenin AE, Adamude FA, Nweze CC, Dingwoke EJ. 2019; Protective effects of Pleurotus ostreatus in ameliorating carbon tetrachloride (ccl4) induced liver injury in Wistar rats. J Med Plants Res. 13:104–11. DOI: 10.5897/JMPR2018.6714.45. Skeggs LT Jr, Hochstrasser H. 1964; Multiple automatic sequential analysis. Clin Chem. 10:918–36. DOI: 10.1093/clinchem/10.10.918. PMID: 14228271.46. Gran FC. 1960; A colorimetric method for the determination of calcium in blood serum. Acta Physiol Scand. 49:192–7. DOI: 10.1111/j.1748-1716.1960.tb01943.x. PMID: 13828859.47. Tuominen VJ, Ruotoistenmäki S, Viitanen A, Jumppanen M, Isola J. 2010; ImmunoRatio: a publicly available web application for quantitative image analysis of estrogen receptor (ER), progesterone receptor (PR), and Ki-67. Breast Cancer Res. 12:R56. DOI: 10.1186/bcr2615. PMID: 20663194. PMCID: PMC2949645.48. 2014. Mar. 31. World health statistics [Internet]. World Health Organization;Available from: https://www.who.int/docs/default-source/gho-documents/world-health-statistic-reports/world-health-statistics-2014.pdf. cited 2024 Mar 12.49. Olokoba AB, Obateru OA, Olokoba LB. 2012; Type 2 diabetes mellitus: a review of current trends. Oman Med J. 27:269–73. DOI: 10.5001/omj.2012.68. PMID: 23071876. PMCID: PMC3464757.50. Galaviz KI, Narayan KMV, Lobelo F, Weber MB. 2015; Lifestyle and the prevention of type 2 diabetes: a status report. Am J Lifestyle Med. 12:4–20. DOI: 10.1177/1559827615619159. PMID: 30202378. PMCID: PMC6125024.51. Fazal F, Shahani HA, Gondal MF, Tanveer U, Haider M, Us Sabah N, Shahzad F, Ur Rehman ME. 2023; Attitudes and factors determining the practice of routine medical checkups in the people of Rawalpindi, Pakistan: a cross-sectional study. Cureus. 15:e38843. DOI: 10.7759/cureus.38843. PMID: 37303352. PMCID: PMC10256253.52. Ojong IN, Nsemo AD, Aji P. 2020; Routine medical checkup knowledge, attitude and practice among health care workers in a tertiary health facility in Calabar, Cross River State, Nigeria. Glob J Health Sci. 12:27–37. DOI: 10.5539/gjhs.v12n8p27.53. Oshio T, Kan M. 2019; Educational level as a predictor of the incidences of non-communicable diseases among middle-aged Japanese: a hazards-model analysis. BMC Public Health. 19:852. DOI: 10.1186/s12889-019-7182-6. PMID: 31262277. PMCID: PMC6604183.54. Adijat OA, Folakemi E, Adejumo A, Atolagbe JE. 2021; The prevalence and risk factors of diabetes mellitus among civil service workers in Osogbo, Osun State, Nigeria. J Hypertens Manag. 7:062. DOI: 10.23937/2474-3690/1510062.55. Tella EE, Yunusa I, Hassan JH, Chindo IA, Oti VB. 2021; Prevalence, contributing factors and management strategies (self-management education) of type 2 diabetes patients in Nigeria: a review. Int J Diabetes Clin Res. 8:148. DOI: 10.23937/2377-3634/1410148.56. Ibrahim MA, Habila JD, Koorbanally NA, Islam MS. 2016; Butanol fraction of Parkia biglobosa (Jacq.) G. Don leaves enhance pancreatic β-cell functions, stimulates insulin secretion and ameliorates other type 2 diabetes-associated complications in rats. J Ethnopharmacol. 183:103–11. DOI: 10.1016/j.jep.2016.02.018. PMID: 26911526.57. Okon U, Owo D, Udokang N, Udobang J, Ekpenyong C. 2012; Oral administration of aqueous leaf extract of Ocimum gratissimum ameliorates polyphagia, polydipsia and weight loss in streptozotocin-induced diabetic rats. Am J Med Med Sci. 2:45–9. DOI: 10.5923/j.ajmms.20120203.04.58. Bindhu MR, Sathe V, Umadevi M. 2013; Synthesis, characterization and SERS activity of biosynthesized silver nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc. 115:409–15. DOI: 10.1016/j.saa.2013.06.047. PMID: 23867642.59. Dawang NS, AbdulHameed A, Ezra GA. 2010; Phytodiversity of three habitat types in Amurum forest: an important bird area in Jos, Nigeria. Afr J Nat Sci. 13:85–94.60. Akunna GG, Saalu LC, Ogunlade B, Akingbade AM. 2013; Tackling infertility with medicinal plant: another instance. Glob J Med Plant Res. 1:93–105.61. Chen RR, Liu ZK, Liu F, Ng TB. 2015; Antihyperglycaemic mechanisms of an aceteoside polymer from rose flowers and a polysaccharide-protein complex from abalone mushroom. Nat Prod Res. 29:558–61. DOI: 10.1080/14786419.2014.952230. PMID: 25200621.62. Erukainure OL, Okafor O, Ajayi A, Obode O, Ogunji A, Okporua T, Suberu Y, Oke O, Ozumba A, Oluwole O, Elemo G. 2015; Developed beverage from roselle calyx and selected fruits modulates β-cell function, improves insulin sensitivity, and attenuates hyperlipidaemia in diabetic rats. Beni-Suef Univ J Basic Appl Sci. 4:307–13. DOI: 10.1016/j.bjbas.2015.11.007.63. Oboh H, Obahiagbon F, Osagie A, Omotosho A. 2011; Glycemic response of some local Nigerian drinks in healthy subjects. Niger J Nutr Sci. 32:79–84. DOI: 10.4314/njns.v32i1.67819.64. Clore JN, Stillman J, Sugerman H. 2000; Glucose-6-phosphatase flux in vitro is increased in type 2 diabetes. Diabetes. 49:969–74. DOI: 10.2337/diabetes.49.6.969. PMID: 10866049.65. Guo X, Li H, Xu H, Woo S, Dong H, Lu F, Lange AJ, Wu C. 2012; Glycolysis in the control of blood glucose homeostasis. Acta Pharm Sin B. 2:358–67. DOI: 10.1016/j.apsb.2012.06.002.66. Islam MS, Choi H. 2007; Green tea, anti-diabetic or diabetogenic: a dose response study. Biofactors. 29:45–53. DOI: 10.1002/biof.5520290105. PMID: 17611293.67. Esterbauer H, Schaur RJ, Zollner H. 1991; Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 11:81–128. DOI: 10.1016/0891-5849(91)90192-6. PMID: 1937131.68. Kangralkar VA, Patil SD, Bandivadekar RM. 2010; Oxidative stress and diabetes: a review. Int J Pharm Appl. 1:38–45.69. Landis GN, Tower J. 2005; Superoxide dismutase evolution and life span regulation. Mech Ageing Dev. 126:365–79. DOI: 10.1016/j.mad.2004.08.012. PMID: 15664623.70. Chelikani P, Fita I, Loewen PC. 2004; Diversity of structures and properties among catalases. Cell Mol Life Sci. 61:192–208. DOI: 10.1007/s00018-003-3206-5. PMID: 14745498. PMCID: PMC11138816.71. AlSaadi BH, AlHarbi SH, Ibrahim SRM, El-Kholy AA, El-Agamy DS, Mohamed GA. 2018; Hepatoprotective activity of costus speciosus (Koen ex. Retz.) against paracetamol-induced liver injury in mice. Afr J Tradit Complement Altern Med. 15:35–41. DOI: 10.21010/ajtcamv15i2.5.72. Ezemagu UK, Akunna GG, Egwu OA, Uzomba GC, Nwite KN. 2019; Comparing Ficus vogelii leaf extract and Omeprazole as therapy and prophylaxis for aspirin-induced gastric ulcer in Wistar rat. Biomed Res. 30:697–703. DOI: 10.35841/biomedicalresearch.30-19-195.73. Hong X, Chi Z, Liu G, Huang H, Guo S, Fan J, Lin X, Qu L, Chen R, Wu L, Wang L, Zhang Q, Wu S, Pan Z, Lin H, Zhou Y, Zhang Y. 2020; Characteristics of renal function in patients diagnosed with COVID-19: an observational study. Front Med. 7:409. DOI: 10.3389/fmed.2020.00409. PMID: 32754610. PMCID: PMC7365839.74. Dere E, Polat F. 2001; The effect of paraquat on the activity of some enzymes in different tissues of mice (Mus musculus - Swiss albino). Turk J Biol. 25:323–32.75. Vuksa M, Nesković N, Vitorović S, Karan V. 1983; Subacute toxicity of paraquat in rats--biochemical effects. Ecotoxicol Environ Saf. 7:475–83. DOI: 10.1016/0147-6513(83)90087-8. PMID: 6641584.76. Attia AM, Nasr HM. 2009; Dimethoate-induced changes in biochemical parameters of experimental rat serum and its neutralization by black seed (Nigella sativa L.) oil. Slovak J Anim Sci. 42:87–94.77. Samai M, Samai HH, Hague T, Naughton D, Chatterjee PK. 2010; Novel superoxide dismutase mimetics for protection against Paraquat-induced acute renal injury. Sierra Leone J Biomed Res. 2:54–64. DOI: 10.4314/sljbr.v2i1.56608.78. Akinloye OA, Adamson I, Ademuyiwa O, Arowolo TA. 2011; Supplementation of vitamins C, E and its combination on paraquat-intoxicated rats: effects on some biochemical and markers of oxidative stress parameters. J Appl Pharm Sci. 1:85–91.79. Smith GL, Shlipak MG, Havranek EP, Foody JM, Masoudi FA, Rathore SS, Krumholz HM. 2006; Serum urea nitrogen, creatinine, and estimators of renal function: mortality in older patients with cardiovascular disease. Arch Intern Med. 166:1134–42. DOI: 10.1001/archinte.166.10.1134. PMID: 16717177.80. Karami S, Yanik EL, Moore LE, Pfeiffer RM, Copeland G, Gonsalves L, Hernandez BY, Lynch CF, Pawlish K, Engels EA. 2016; Risk of renal cell carcinoma among kidney transplant recipients in the United States. Am J Transplant. 16:3479–89. DOI: 10.1111/ajt.13862. PMID: 27160653. PMCID: PMC5104677.81. Zhao S, Ghosh A, Lo CS, Chenier I, Scholey JW, Filep JG, Ingelfinger JR, Zhang SL, Chan JSD. 2018; Nrf2 deficiency upregulates intrarenal angiotensin-converting enzyme-2 and angiotensin 1-7 receptor expression and attenuates hypertension and nephropathy in diabetic mice. Endocrinology. 159:836–52. DOI: 10.1210/en.2017-00752. PMID: 29211853. PMCID: PMC5774246.82. Heiss EH, Schachner D, Werner ER, Dirsch VM. 2009; Active NF-E2-related factor (Nrf2) contributes to keep endothelial NO synthase (eNOS) in the coupled state: role of reactive oxygen species (ROS), eNOS, and heme oxygenase (HO-1) levels. J Biol Chem. 284:31579–86. DOI: 10.1074/jbc.M109.009175. PMID: 19797052. PMCID: PMC2797228.83. Aminzadeh MA, Nicholas SB, Norris KC, Vaziri ND. 2013; Role of impaired Nrf2 activation in the pathogenesis of oxidative stress and inflammation in chronic tubulo-interstitial nephropathy. Nephrol Dial Transplant. 28:2038–45. DOI: 10.1093/ndt/gft022. PMID: 23512109. PMCID: PMC3765021.84. Fahey JW, Zalcmann AT, Talalay P. 2001; The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 56:5–51. DOI: 10.1016/S0031-9422(00)00316-2. PMID: 11198818.85. Rushmore TH, Kong AN. 2002; Pharmacogenomics, regulation and signaling pathways of phase I and II drug metabolizing enzymes. Curr Drug Metab. 3:481–90. DOI: 10.2174/1389200023337171. PMID: 12369894.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Therapeutic Effect of Amomum xanthoides Extract on Experimental Diabetes Induced by Alloxan
- The protective effect of Amomum xanthoides extract against alloxan-induced diabetes through the suppression of NFkappaB activation
- Efficient Recovery of Lignocellulolytic Enzymes of Spent Mushroom Compost from Oyster Mushrooms, Pleurotus spp., and Potential Use in Dye Decolorization
- Isolation of Fungal Pathogens to an Edible Mushroom, Pleurotus eryngii, and Development of Specific ITS Primers
- Isolation of Genes Specifically Expressed in Different Developmental Stages of Pleurotus ostreatus Using Macroarray Analysis