Clin Endosc.
2024 Sep;57(5):637-646. 10.5946/ce.2024.027.
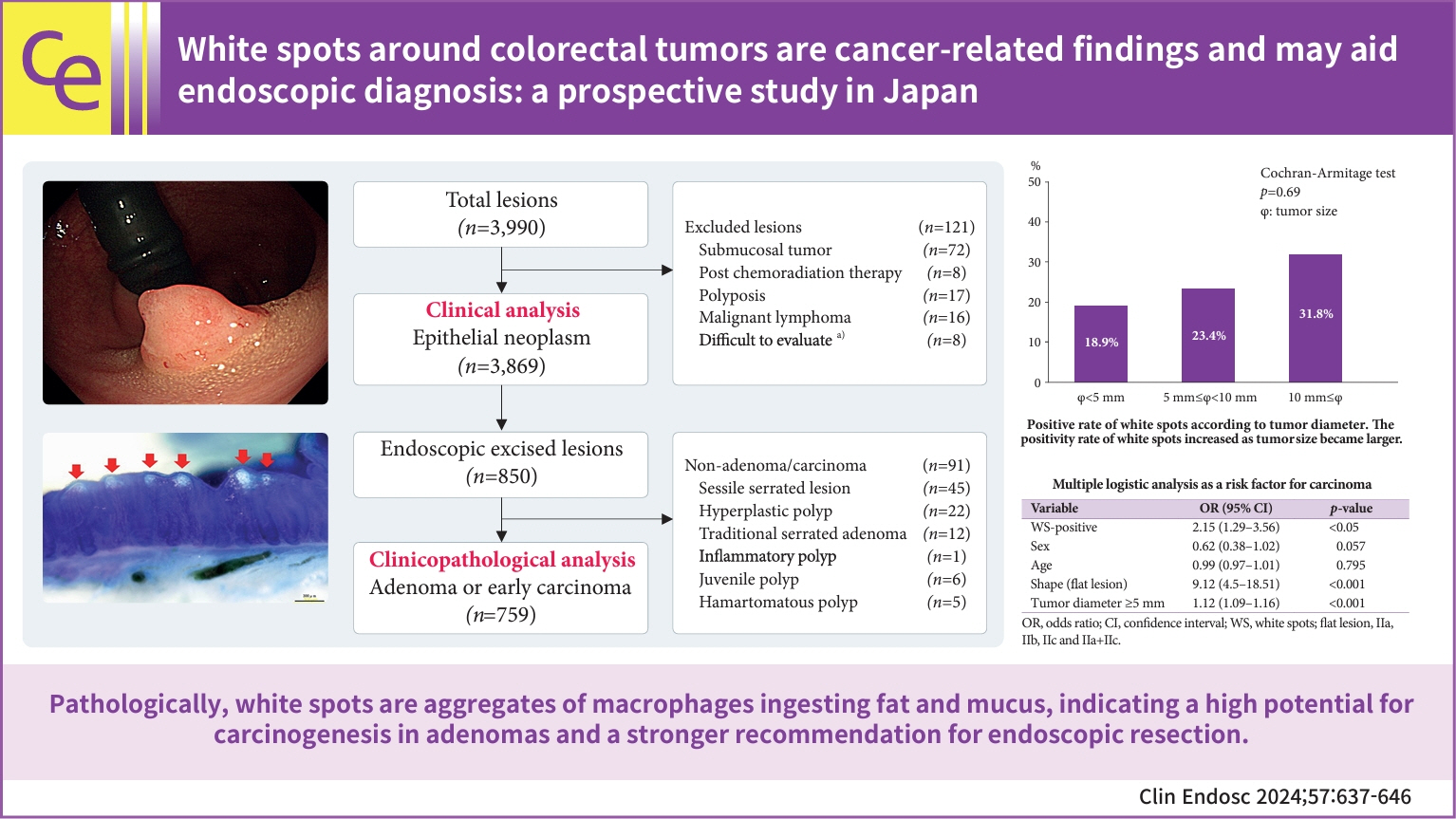
White spots around colorectal tumors are cancer-related findings and may aid endoscopic diagnosis: a prospective study in Japan
- Affiliations
-
- 1Division of Gastroenterology, Tohoku University Graduate School of Medicine, Sendai, Japan
- 2Department of Pathology, Tohoku University Graduate School of Medicine, Sendai, Japan
- 3Student Healthcare Center, Institute for Excellence in Higher Education, Tohoku University, Sendai, Japan
- KMID: 2559215
- DOI: http://doi.org/10.5946/ce.2024.027
Abstract
- Background/Aims
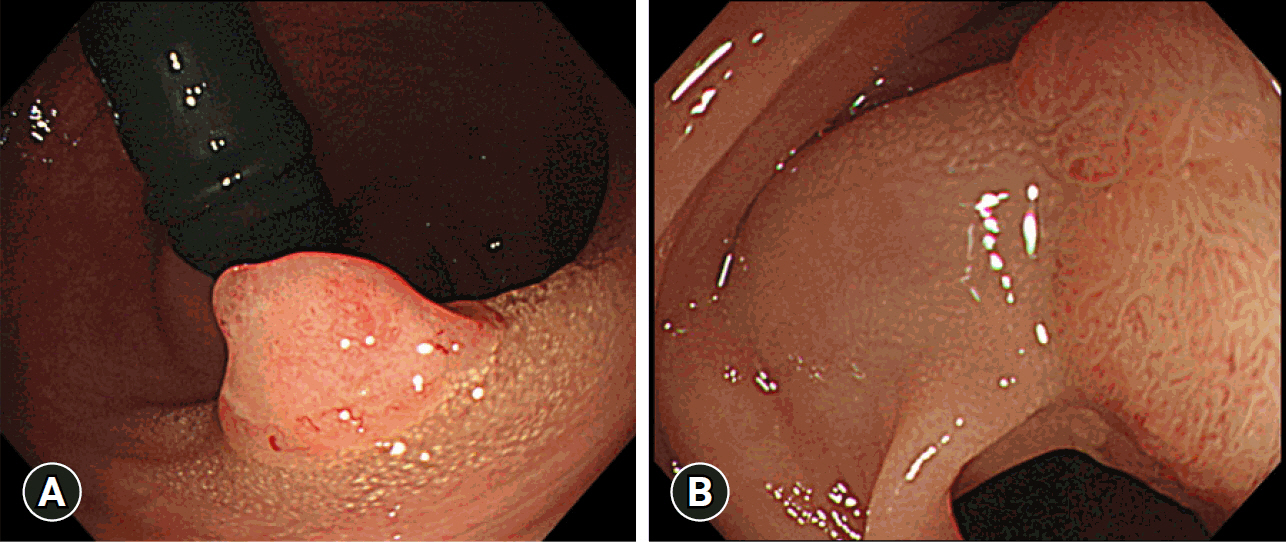
During endoscopy, white spots (WS) are sometimes observed around benign or malignant colorectal tumors; however, few reports have investigated WS, and their significance remains unknown. Therefore, we investigated the significance of WS from clinical and pathological viewpoints and evaluated its usefulness in endoscopic diagnosis.
Methods
Clinical data of patients with lesions diagnosed as epithelial tumors from January 1, 2019, to December 31, 2020, were analyzed (n=3,869). We also performed a clinicopathological analysis of adenomas or carcinomas treated with endoscopic resection (n=759). Subsequently, detailed pathological observations of the WS were performed.
Results
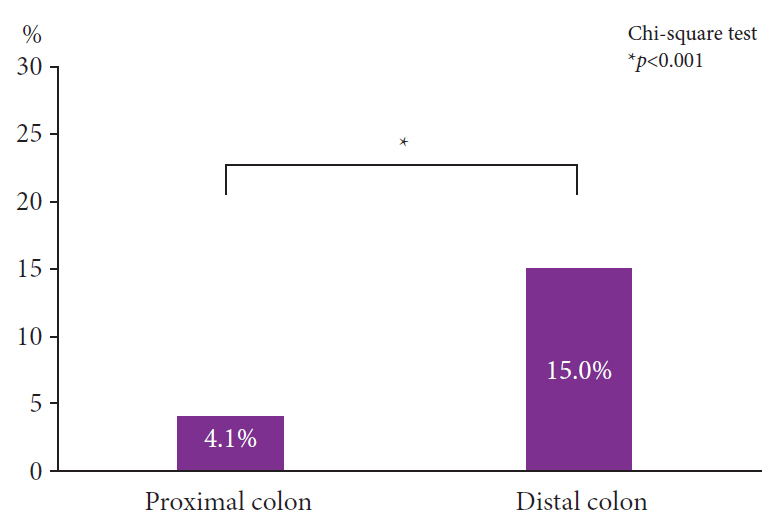
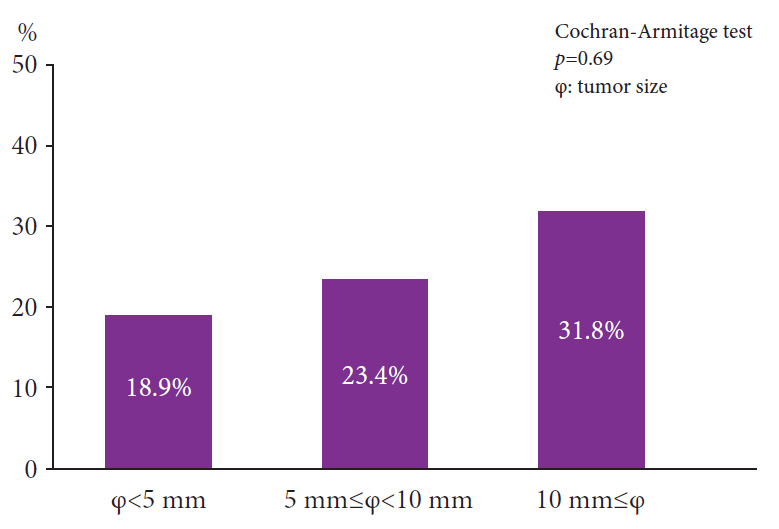
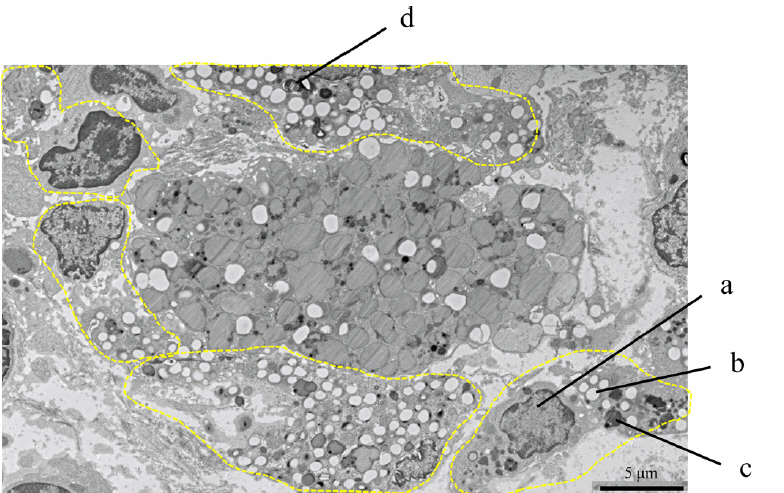
The positivity rates for WS were 9.3% (3,869 lesions including advanced cancer and non-adenoma/carcinoma) and 25% (759 lesions limited to adenoma and early carcinoma). Analysis of 759 lesions showed that the WS-positive lesion group had a higher proportion of cancer cases and larger tumor diameters than the WS-negative group. Multiple logistic analysis revealed the following three statistically significant risk factors for carcinogenesis: positive WS, flat lesions, and tumor diameter ≥5 mm. Pathological analysis revealed that WS were macrophages that phagocytosed fat and mucus and were white primarily because of fat.
Conclusions
WS are cancer-related findings and can become a new criterion for endoscopic resection in the future.
Figure
Reference
-
1. Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021; 325:669–685.2. Hill MJ, Morson BC, Bussey HJ. Aetiology of adenoma: carcinoma sequence in large bowel. Lancet. 1978; 1:245–247.3. Goto H, Oda Y, Murakami Y, et al. Proportion of de novo cancers among colorectal cancers in Japan. Gastroenterology. 2006; 131:40–46.4. Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010; 138:2088–2100.5. Jass JR, Whitehall VL, Young J, et al. Emerging concepts in colorectal neoplasia. Gastroenterology. 2002; 123:862–876.6. Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012; 366:687–696.7. Chung EJ, Lee JY, Choe J, et al. Colonic chicken skin mucosa is an independent endoscopic predictor of advanced colorectal adenoma. Intest Res. 2015; 13:318–325.8. Lee YM, Song KH, Koo HS, et al. Colonic chicken skin mucosa surrounding colon polyps is an endoscopic predictive marker for colonic neoplastic polyps. Gut Liver. 2022; 16:754–763.9. Zhang YJ, Wen W, Li F, et al. Chicken skin mucosa surrounding small colorectal cancer could be an endoscopic predictive marker of submucosal invasion. World J Gastrointest Oncol. 2023; 15:1062–1072.10. Muto T, Kamiya J, Sawada T, et al. Clinical and histological studies on white spots of colonic mucosa around colonic polyps with special reference to diagnosis of early carcinoma. Gastroenterol Endosc. 1981; 23:241–247.11. Hirota S, Hosobe S, Ikeda M, et al. Possibility of defense against colorectal tumor by foamy cells. J Clin Gastroenterol. 1997; 24:82–86.12. Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014; 370:1298–1306.13. van Doorn SC, Klanderman RB, Hazewinkel Y, et al. Adenoma detection rate varies greatly during colonoscopy training. Gastrointest Endosc. 2015; 82:122–129.14. Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2015; 110:72–90.15. Tanaka S, Saitoh Y, Matsuda T, et al. Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol. 2015; 50:252–260.16. Tanaka S, Saitoh Y, Matsuda T, et al. Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol. 2021; 56:323–335.17. Lambert R, Lightdale CJ. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon. Gastrointest Endosc. 2003; 58:S3–S43.18. Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000; 47:251–255.19. Schlemper RJ, Kato Y, Stolte M. Review of histological classifications of gastrointestinal epithelial neoplasia: differences in diagnosis of early carcinomas between Japanese and Western pathologists. J Gastroenterol. 2001; 36:445–456.20. Sakamoto T, Matsuda T, Nakajima T, et al. Clinicopathological features of colorectal polyps: evaluation of the ‘predict, resect and discard’ strategies. Colorectal Dis. 2013; 15:e295–e300.21. Gschwantler M, Kriwanek S, Langner E, et al. High-grade dysplasia and invasive carcinoma in colorectal adenomas: a multivariate analysis of the impact of adenoma and patient characteristics. Eur J Gastroenterol Hepatol. 2002; 14:183–188.22. O’Brien MJ, Winawer SJ, Zauber AG, et al. The National Polyp Study. Patient and polyp characteristics associated with high-grade dysplasia in colorectal adenomas. Gastroenterology. 1990; 98:371–379.23. Lieberman DA, Weiss DG, Harford WV, et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology. 2007; 133:1077–1085.24. Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006; 66:605–612.25. Wagener S, Shankar KR, Turnock RR, et al. Colonic transit time: what is normal? J Pediatr Surg. 2004; 39:166–169.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Histopathological Features and the Clinical Significance of White Spots around Colon Cancer
- Endoscopic diagnosis and treatment of early colorectal cancer
- White Opaque Substance, a New Optical Marker on Magnifying Endoscopy: Usefulness in Diagnosing Colorectal Epithelial Neoplasms
- Endoscopic Diagnosis and Treatment of Early Colorectal Cancer
- Advanced Treatment and Imaging in Colonoscopy: The Pocket-Creation Method for Complete Resection and Linked Color Imaging for Better Detection of Early Neoplastic Lesions by Colonoscopy