Korean J Physiol Pharmacol.
2024 Sep;28(5):413-422. 10.4196/kjpp.2024.28.5.413.
Roles of metabotropic glutamate receptor 5 in low [Mg2+ ]o -induced interictal epileptiform activity in rat hippocampal slices
- Affiliations
-
- 1Department of Physiology, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea
- 2Catholic Neuroscience Institute, The Catholic University of Korea, Seoul 06591, Korea
- 3Department of Pharmacology, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea
- KMID: 2559134
- DOI: http://doi.org/10.4196/kjpp.2024.28.5.413
Abstract
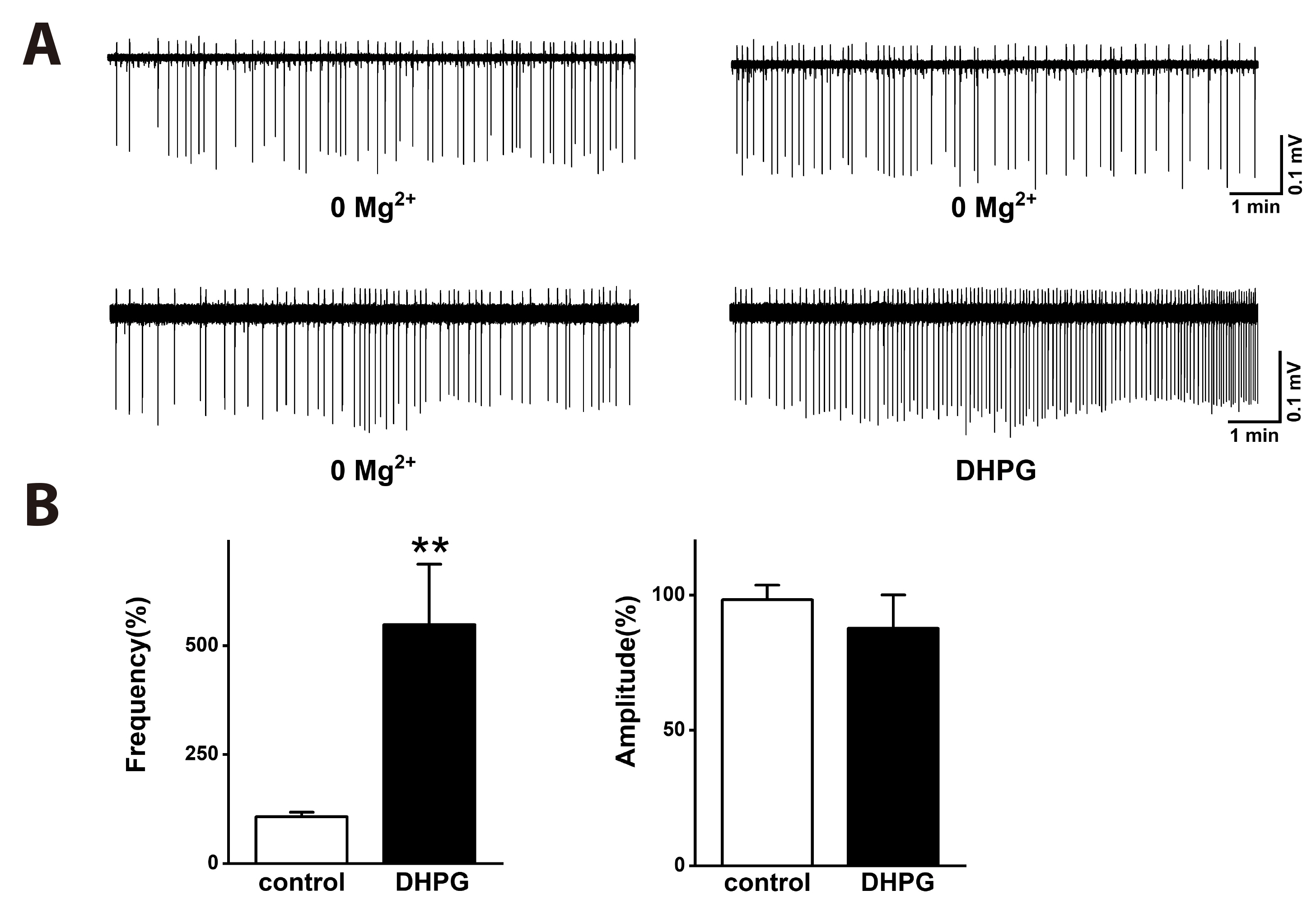
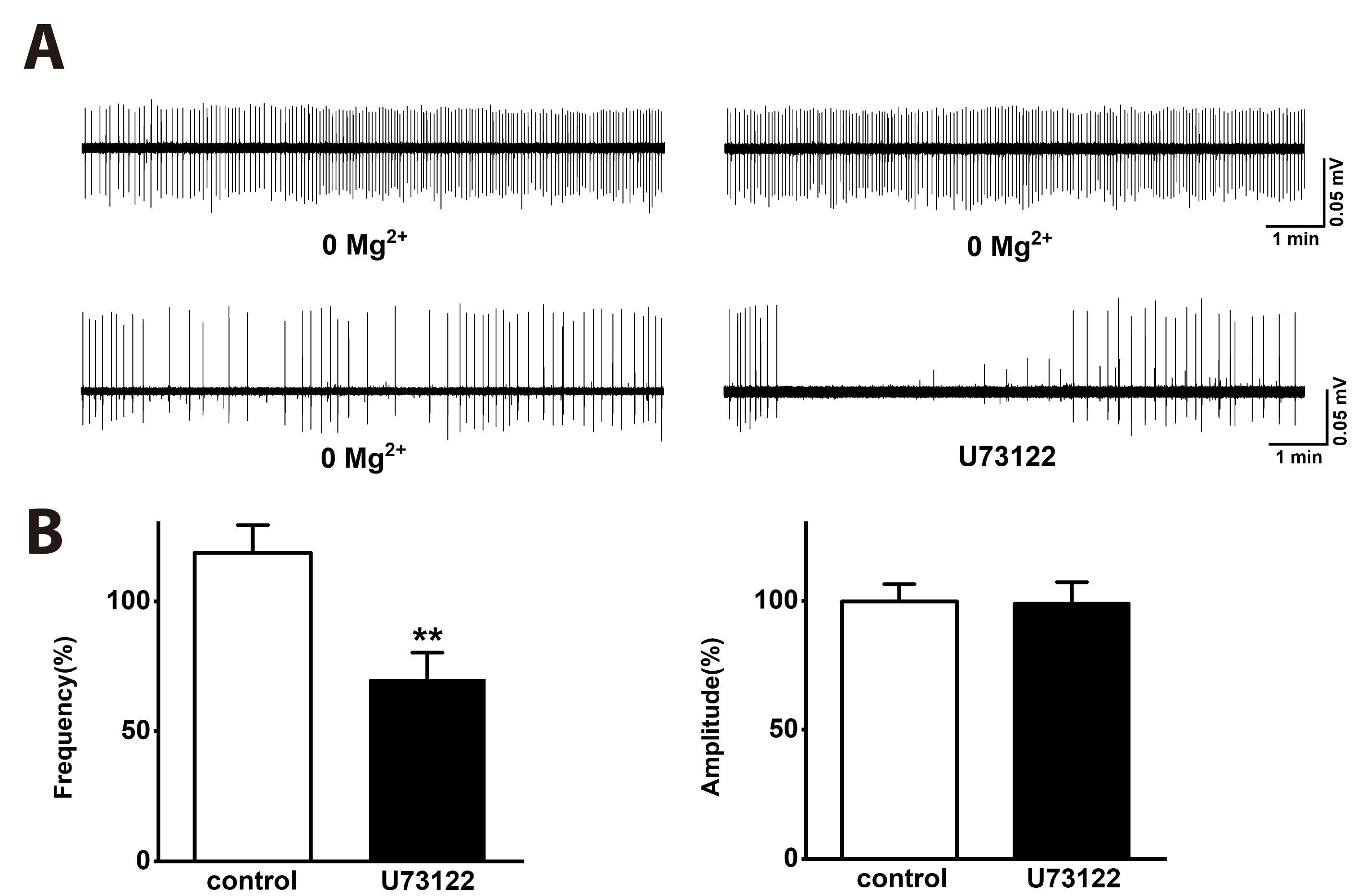
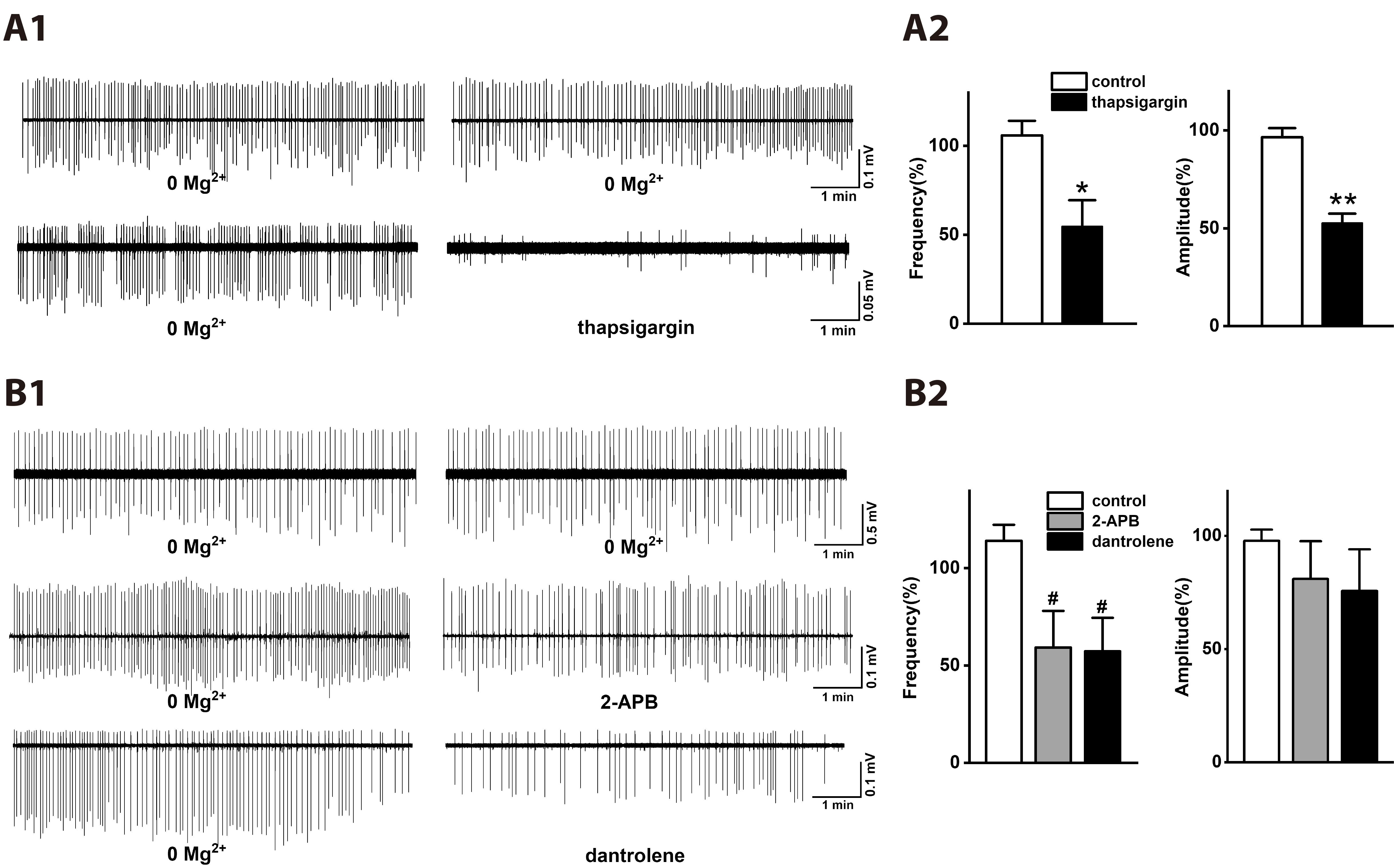
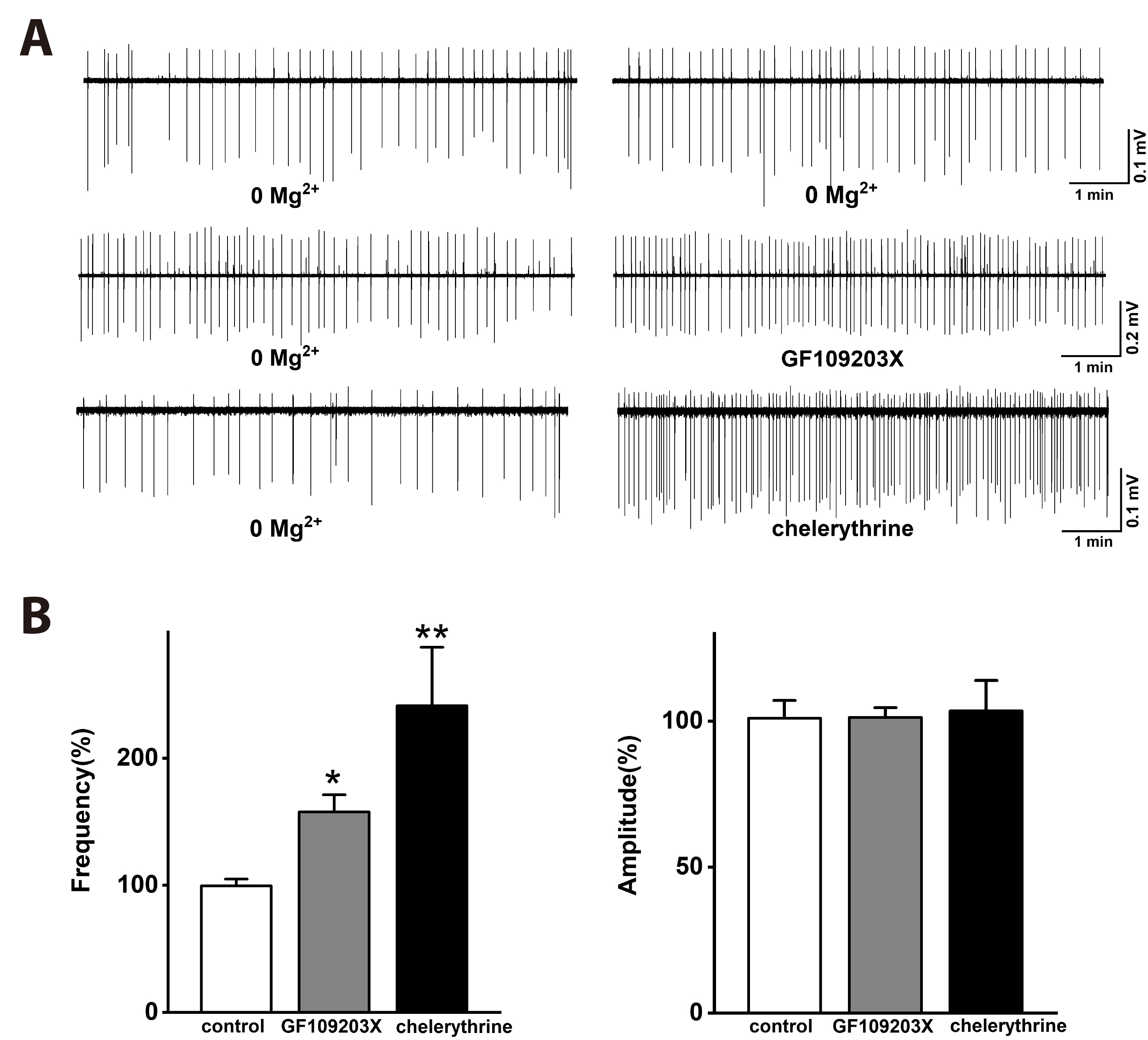
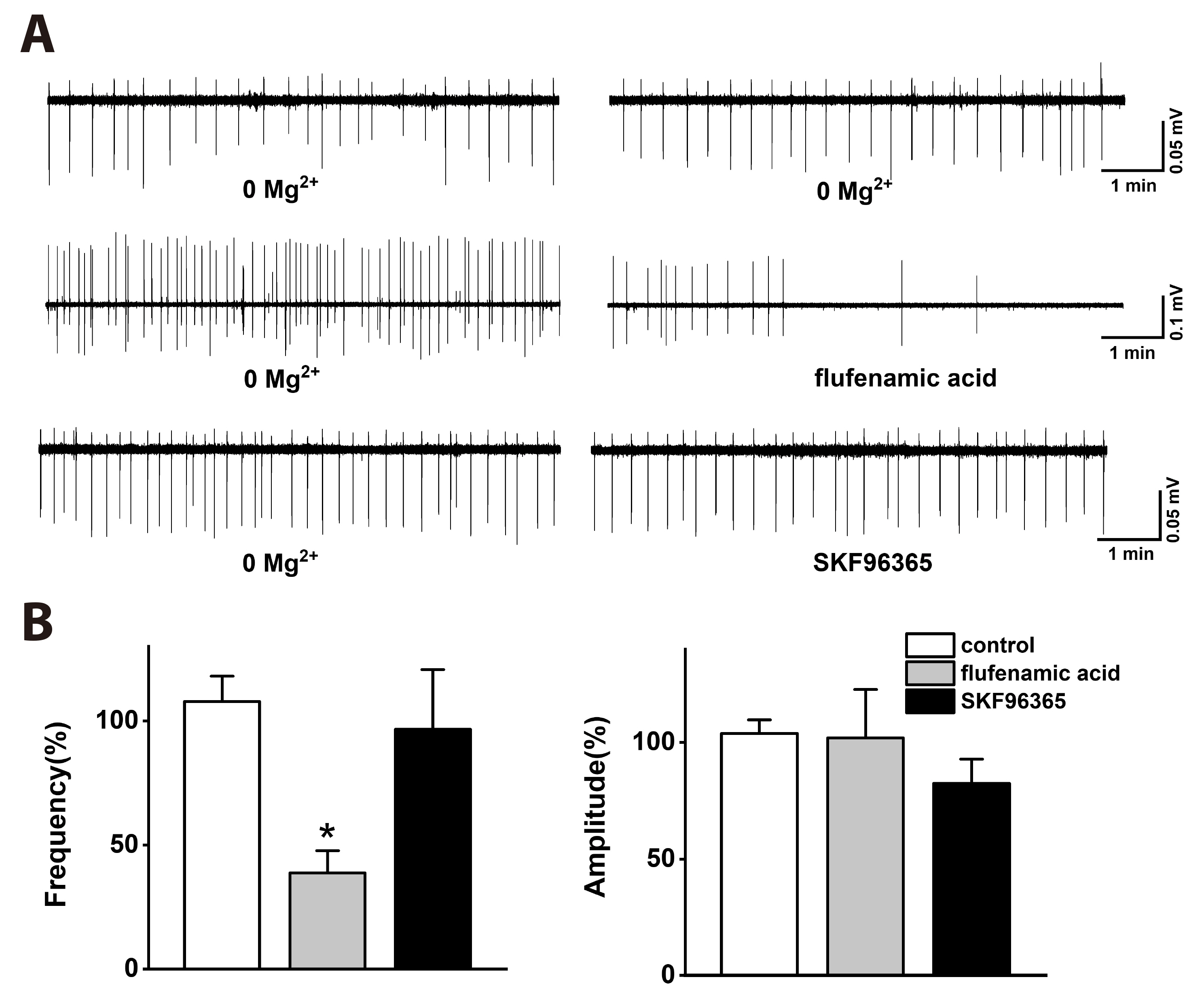
- Group I metabotropic glutamate receptors (mGluRs) modulate postsynaptic neuronal excitability and epileptogenesis. We investigated roles of group I mGluRs on low extracellular Mg2+ concentration ([Mg2+ ]o )-induced epileptiform activity and neuronal cell death in the CA1 regions of isolated rat hippocampal slices without the entorhinal cortex using extracellular recording and propidium iodide staining. Exposure to Mg2+ -free artificial cerebrospinal fluid can induce interictal epileptiform activity in the CA1 regions of rat hippocampal slices. MPEP, a mGluR 5 antagonist, significantly inhibited the spike firing of the low [Mg2+ ]o -induced epileptiform activity, whereas LY367385, a mGluR1 antagonist, did not. DHPG, a group 1 mGluR agonist, significantly increased the spike firing of the epileptiform activity. U73122, a PLC inhibitor, inhibited the spike firing. Thapsigargin, an ER Ca2+ -ATPase antagonist, significantly inhibited the spike firing and amplitude of the epileptiform activity. Both the IP 3 receptor antagonist 2-APB and the ryanodine receptor antagonist dantrolene significantly inhibited the spike firing. The PKC inhibitors such as chelerythrine and GF109203X, significantly increased the spike firing. Flufenamic acid, a relatively specific TRPC 1, 4, 5 channel antagonist, significantly inhibited the spike firing, whereas SKF96365, a relatively non-specific TRPC channel antagonist, did not. MPEP significantly decreased low [Mg2+ ] o DMEM-induced neuronal cell death in the CA1 regions, but LY367385 did not. We suggest that mGluR 5 is involved in low [Mg2+ ]o -induced interictal epileptiform activity in the CA1 regions of rat hippocampal slices through PLC, release of Ca2+ from intracellular stores and PKC and TRPC channels, which could be involved in neuronal cell death.
Keyword
Figure
Reference
-
1. Abele AE, Scholz KP, Scholz WK, Miller RJ. 1990; Excitotoxicity induced by enhanced excitatory neurotransmission in cultured hippocampal pyramidal neurons. Neuron. 4:413–419. DOI: 10.1016/0896-6273(90)90053-I. PMID: 1690567.2. Shen M, Piser TM, Seysub VS, Thayer SA. 1996; Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. J Neurosci. 16:4322–4334. DOI: 10.1523/JNEUROSCI.16-14-04322.1996. PMID: 8699243. PMCID: PMC6578864.3. Mody I, Lambert JD, Heinemann U. 1987; Low extracellular magnesium induces epileptiform activity and spreading depression in rat hippocampal slices. J Neurophysiol. 57:869–888. DOI: 10.1152/jn.1987.57.3.869. PMID: 3031235.4. Tancredi V, Hwa GG, Zona C, Brancati A, Avoli M. 1990; Low magnesium epileptogenesis in the rat hippocampal slice: electrophysiological and pharmacological features. Brain Res. 511:280–290. DOI: 10.1016/0006-8993(90)90173-9. PMID: 1970748.5. Traub RD, Jefferys JG, Whittington MA. 1994; Enhanced NMDA conductance can account for epileptiform activity induced by low Mg2+ in the rat hippocampal slice. J Physiol. 478(Pt 3):379–393. DOI: 10.1113/jphysiol.1994.sp020259. PMID: 7965853. PMCID: PMC1155660.6. Raimondo JV, Heinemann U, de Curtis M, Goodkin HP, Dulla CG, Janigro D, Ikeda A, Lin CK, Jiruska P, Galanopoulou AS, Bernard C. 2017; Methodological standards for in vitro models of epilepsy and epileptic seizures. A TASK1-WG4 report of the AES/ILAE Translational Task Force of the ILAE. Epilepsia. 58 Suppl 4(Suppl 4):40–52. DOI: 10.1111/epi.13901. PMID: 29105075. PMCID: PMC5679463.7. Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. 1997; Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 17:7503–7522. DOI: 10.1523/JNEUROSCI.17-19-07503.1997. PMID: 9295396. PMCID: PMC6573434.8. Niswender CM, Conn PJ. 2010; Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 50:295–322. DOI: 10.1146/annurev.pharmtox.011008.145533. PMID: 20055706. PMCID: PMC2904507.9. El-Hassar L, Hagenston AM, D'Angelo LB, Yeckel MF. 2011; Metabotropic glutamate receptors regulate hippocampal CA1 pyramidal neuron excitability via Ca²+ wave-dependent activation of SK and TRPC channels. J Physiol. 589:3211–3229. DOI: 10.1113/jphysiol.2011.209783. PMID: 21576272. PMCID: PMC3145935.10. Merlin LR, Wong RK. 1997; Role of group I metabotropic glutamate receptors in the patterning of epileptiform activities in vitro. J Neurophysiol. 78:539–544. DOI: 10.1152/jn.1997.78.1.539. PMID: 9242303.11. Pan YZ, Rutecki PA. 2014; Enhanced excitatory synaptic network activity following transient group I metabotropic glutamate activation. Neuroscience. 275:22–32. DOI: 10.1016/j.neuroscience.2014.05.062. PMID: 24928353.12. Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P. 1996; Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci. 8:1488–1500. DOI: 10.1111/j.1460-9568.1996.tb01611.x. PMID: 8758956.13. Lepannetier S, Gualdani R, Tempesta S, Schakman O, Seghers F, Kreis A, Yerna X, Slimi A, de Clippele M, Tajeddine N, Voets T, Bon RS, Beech DJ, Tissir F, Gailly P. 2018; Activation of TRPC1 channel by metabotropic glutamate receptor mGluR5 modulates synaptic plasticity and spatial working memory. Front Cell Neurosci. 12:318. DOI: 10.3389/fncel.2018.00318. PMID: 30271326. PMCID: PMC6149316.14. Gualdani R, Gailly P. 2020; How TRPC channels modulate hippocampal function. Int J Mol Sci. 21:3915. DOI: 10.3390/ijms21113915. PMID: 32486187. PMCID: PMC7312571.15. Nakatsuka H, Natsume K. 2014; Circadian rhythm modulates long-term potentiation induced at CA1 in rat hippocampal slices. Neurosci Res. 80:1–9. DOI: 10.1016/j.neures.2013.12.007. PMID: 24406747.16. Avoli M, D'Antuono M, Louvel J, Köhling R, Biagini G, Pumain R, D'Arcangelo G, Tancredi V. 2002; Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog Neurobiol. 68:167–207. DOI: 10.1016/S0301-0082(02)00077-1. PMID: 12450487.17. Stoppini L, Buchs PA, Muller D. 1991; A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 37:173–182. DOI: 10.1016/0165-0270(91)90128-M. PMID: 1715499.18. Happ DF, Tasker RA. 2016; A method for objectively quantifying propidium iodide exclusion in organotypic hippocampal slice cultures. J Neurosci Methods. 269:1–5. DOI: 10.1016/j.jneumeth.2016.05.006. PMID: 27179931.19. Yang JS, Jeon S, Jang HJ, Yoon SH. 2022; Group 1 metabotropic glutamate receptor 5 is involved in synaptically-induced Ca2+-spikes and cell death in cultured rat hippocampal neurons. Korean J Physiol Pharmacol. 26:531–540. DOI: 10.4196/kjpp.2022.26.6.531. PMID: 36302627. PMCID: PMC9614404.20. Nishizuka Y, Shearman MS, Oda T, Berry N, Shinomura T, Asaoka Y, Ogita K, Koide H, Kikkawa U, Kishimoto A, Kose A, Saito N, Tanaka C. 1991; Protein kinase C family and nervous function. Prog Brain Res. 89:125–141. DOI: 10.1016/S0079-6123(08)61719-7. PMID: 1796138.21. Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE. 2001; TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 29:645–655. DOI: 10.1016/S0896-6273(01)00240-9. PMID: 11301024.22. Chung YH, Sun Ahn H, Kim D, Hoon Shin D, Su Kim S, Yong Kim K, Bok Lee W, Ik Cha C. 2006; Immunohistochemical study on the distribution of TRPC channels in the rat hippocampus. Brain Res. 1085:132–137. DOI: 10.1016/j.brainres.2006.02.087. PMID: 16580647.23. Rae MG, Hilton J, Sharkey J. 2012; Putative TRP channel antagonists, SKF 96365, flufenamic acid and 2-APB, are non-competitive antagonists at recombinant human α1β2γ2 GABA(A) receptors. Neurochem Int. 60:543–554. DOI: 10.1016/j.neuint.2012.02.014. PMID: 22369768.24. Kovács R, Gutiérrez R, Kivi A, Schuchmann S, Gabriel S, Heinemann U. 1999; Acute cell damage after low Mg2+-induced epileptiform activity in organotypic hippocampal slice cultures. Neuroreport. 10:207–213. DOI: 10.1097/00001756-199902050-00002. PMID: 10203310.25. Lanneau C, Harries MH, Ray AM, Cobb SR, Randall A, Davies CH. 2002; Complex interactions between mGluR1 and mGluR5 shape neuronal network activity in the rat hippocampus. Neuropharmacology. 43:131–140. DOI: 10.1016/S0028-3908(02)00086-2. PMID: 12213267.26. Simpson PB, Challiss RA, Nahorski SR. 1995; Neuronal Ca2+ stores: activation and function. Trends Neurosci. 18:299–306. DOI: 10.1016/0166-2236(95)93919-O. PMID: 7571010.27. Hagenston AM, Fitzpatrick JS, Yeckel MF. 2008; MGluR-mediated calcium waves that invade the soma regulate firing in layer V medial prefrontal cortical pyramidal neurons. Cereb Cortex. 18:407–423. DOI: 10.1093/cercor/bhm075. PMID: 17573372. PMCID: PMC3005283.28. Thastrup O, Cullen PJ, Drøbak BK, Hanley MR, Dawson AP. 1990; Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 87:2466–2470. DOI: 10.1073/pnas.87.7.2466. PMID: 2138778. PMCID: PMC53710.29. Wülfert E, Margineanu DG. 1998; Thapsigargin inhibits bicuculline-induced epileptiform excitability in rat hippocampal slices. Neurosci Lett. 243:141–143. DOI: 10.1016/S0304-3940(98)00073-1. PMID: 9535133.30. Rutecki PA, Sayin U, Yang Y, Hadar E. 2002; Determinants of ictal epileptiform patterns in the hippocampal slice. Epilepsia. 43 Suppl 5:179–183. DOI: 10.1046/j.1528-1157.43.s.5.34.x. PMID: 12121317.31. Hsu KS, Ho WC, Huang CC, Tsai JJ. 2000; Transient removal of extracellular Mg(2+) elicits persistent suppression of LTP at hippocampal CA1 synapses via PKC activation. J Neurophysiol. 84:1279–1288. DOI: 10.1152/jn.2000.84.3.1279. PMID: 10980002.32. Stanton PK. 1995; Transient protein kinase C activation primes long-term depression and suppresses long-term potentiation of synaptic transmission in hippocampus. Proc Natl Acad Sci U S A. 92:1724–1728. DOI: 10.1073/pnas.92.5.1724. PMID: 7878048. PMCID: PMC42592.33. Cuellar JC, Griffith EL, Merlin LR. 2005; Contrasting roles of protein kinase C in induction versus suppression of group I mGluR-mediated epileptogenesis in vitro. J Neurophysiol. 94:3643–3647. DOI: 10.1152/jn.00548.2005. PMID: 16049142.34. Terunuma M, Xu J, Vithlani M, Sieghart W, Kittler J, Pangalos M, Haydon PG, Coulter DA, Moss SJ. 2008; Deficits in phosphorylation of GABA(A) receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J Neurosci. 28:376–384. DOI: 10.1523/JNEUROSCI.4346-07.2008. PMID: 18184780. PMCID: PMC2917223.35. Kovács R, Szilágyi N, Barabás P, Heinemann U, Kardos J. 2000; Low-[Mg2+]-induced Ca2+ fluctuations in organotypic hippocampal slice cultures. Neuroreport. 11:2107–2111. DOI: 10.1097/00001756-200007140-00010. PMID: 10923653.36. Jiang H, Zeng B, Chen GL, Bot D, Eastmond S, Elsenussi SE, Atkin SL, Boa AN, Xu SZ. 2012; Effect of non-steroidal anti-inflammatory drugs and new fenamate analogues on TRPC4 and TRPC5 channels. Biochem Pharmacol. 83:923–931. DOI: 10.1016/j.bcp.2012.01.014. PMID: 22285229.37. Boulay G, Zhu X, Peyton M, Jiang M, Hurst R, Stefani E, Birnbaumer L. 1997; Cloning and expression of a novel mammalian homolog of Drosophila transient receptor potential (Trp) involved in calcium entry secondary to activation of receptors coupled by the Gq class of G protein. J Biol Chem. 272:29672–29680. DOI: 10.1074/jbc.272.47.29672. PMID: 9368034.38. Shlykov SG, Yang M, Alcorn JL, Sanborn BM. 2003; Capacitative cation entry in human myometrial cells and augmentation by hTrpC3 overexpression. Biol Reprod. 69:647–655. DOI: 10.1095/biolreprod.103.015396. PMID: 12700192.39. Phelan KD, Shwe UT, Abramowitz J, Wu H, Rhee SW, Howell MD, Gottschall PE, Freichel M, Flockerzi V, Birnbaumer L, Zheng F. 2013; Canonical transient receptor channel 5 (TRPC5) and TRPC1/4 contribute to seizure and excitotoxicity by distinct cellular mechanisms. Mol Pharmacol. 83:429–438. DOI: 10.1124/mol.112.082271. PMID: 23188715. PMCID: PMC3558807.40. Strasser U, Lobner D, Behrens MM, Canzoniero LM, Choi DW. 1998; Antagonists for group I mGluRs attenuate excitotoxic neuronal death in cortical cultures. Eur J Neurosci. 10:2848–2855. DOI: 10.1111/j.1460-9568.1998.00291.x. PMID: 9758154.41. Avoli M. 2001; Do interictal discharges promote or control seizures? Experimental evidence from an in vitro model of epileptiform discharge. Epilepsia. 42(Suppl 3):2–4. DOI: 10.1046/j.1528-1157.2001.042suppl.3002.x. PMID: 11520313.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Properties of Low Magnesium Induced Epileptiform Activity in Rat Visual Cortex Slices
- Effect of Potassium, Bicuculline and CNQX on Epileptiform Activity Induced by Pilocarpine in the Rat Visual Cortex Slice
- The Role of Metabotropic Glutamate Receptors in Psychomotor Stimulant Addiction
- Group 1 metabotropic glutamate receptor 5 is involved in synaptically-induced Ca2+ -spikes and cell death in cultured rat hippocampal neurons
- Tetraethylammonium-induced Epileptiform Activity and its Modification by GABAA Antagonist in the Rat Visual Cortex