Endocrinol Metab.
2024 Aug;39(4):552-558. 10.3803/EnM.2024.1989.
Parathyroid Gland Generation from Pluripotent Stem Cells
- Affiliations
-
- 1Department of Metabolism and Endocrinology, St. Marianna University School of Medicine, Kawasaki, Japan
- KMID: 2558937
- DOI: http://doi.org/10.3803/EnM.2024.1989
Abstract
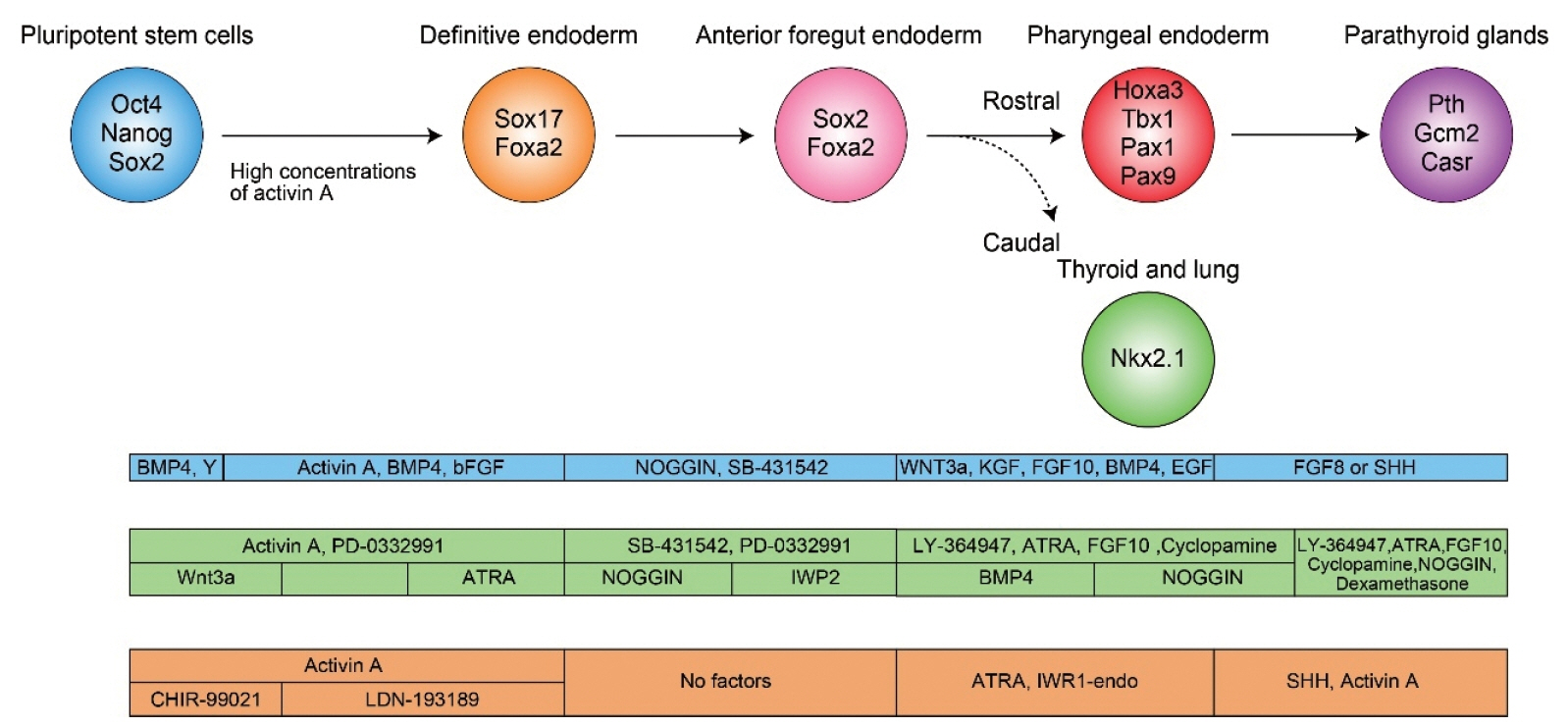
- Patients with permanent hypoparathyroidism require lifelong treatment. Current replacement therapies sometimes have adverse effects (e.g., hypercalciuria and chronic kidney disease). Generating parathyroid glands (PTGs) from the patient’s own induced pluripotent stem cells (PSCs), with transplantation of these PTGs, would be an effective treatment option. Multiple methods for generating PTGs from PSCs have been reported. One major trend is in vitro differentiation of PSCs into PTGs. Another is in vivo generation of PSC-derived PTGs by injecting PSCs into PTG-deficient embryos. This review discusses current achievements and challenges in present and future PTG regenerative medicine.
Figure
Reference
-
1. Mitchell DM, Regan S, Cooley MR, Lauter KB, Vrla MC, Becker CB, et al. Long-term follow-up of patients with hypoparathyroidism. J Clin Endocrinol Metab. 2012; 97:4507–14.
Article2. Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol. 2009; 25:221–51.
Article3. Graham A. Development of the pharyngeal arches. Am J Med Genet A. 2003; 119A:251–6.
Article4. Grevellec A, Tucker AS. The pharyngeal pouches and clefts: development, evolution, structure and derivatives. Semin Cell Dev Biol. 2010; 21:325–32.
Article5. Jerome LA, Papaioannou VE. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat Genet. 2001; 27:286–91.
Article6. Gunther T, Chen ZF, Kim J, Priemel M, Rueger JM, Amling M, et al. Genetic ablation of parathyroid glands reveals another source of parathyroid hormone. Nature. 2000; 406:199–203.
Article7. Liu Z, Yu S, Manley NR. Gcm2 is required for the differentiation and survival of parathyroid precursor cells in the parathyroid/thymus primordia. Dev Biol. 2007; 305:333–46.
Article8. Su D, Ellis S, Napier A, Lee K, Manley NR. Hoxa3 and pax1 regulate epithelial cell death and proliferation during thymus and parathyroid organogenesis. Dev Biol. 2001; 236:316–29.
Article9. Peters H, Neubuser A, Kratochwil K, Balling R. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 1998; 12:2735–47.10. Green MD, Chen A, Nostro MC, d’Souza SL, Schaniel C, Lemischka IR, et al. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat Biotechnol. 2011; 29:267–72.
Article11. Moore-Scott BA, Manley NR. Differential expression of Sonic hedgehog along the anterior-posterior axis regulates patterning of pharyngeal pouch endoderm and pharyngeal endoderm-derived organs. Dev Biol. 2005; 278:323–35.
Article12. Gordon J, Manley NR. Mechanisms of thymus organogenesis and morphogenesis. Development. 2011; 138:3865–78.
Article13. Lawton BR, Martineau C, Sosa JA, Roman S, Gibson CE, Levine MA, et al. Differentiation of PTH-expressing cells from human pluripotent stem cells. Endocrinology. 2020; 161:bqaa141.14. Nakatsuka R, Kato T, Zhang R, Uemura Y, Sasaki Y, Matsuoka Y, et al. The induction of parathyroid cell differentiation from human induced pluripotent stem cells promoted via TGF-α/EGFR signaling. Stem Cells Dev. 2023; 32:670–80.
Article15. D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006; 24:1392–401.
Article16. Huang SX, Green MD, de Carvalho AT, Mumau M, Chen YW, D’Souza SL, et al. The in vitro generation of lung and airway progenitor cells from human pluripotent stem cells. Nat Protoc. 2015; 10:413–25.
Article17. Patel SR, Gordon J, Mahbub F, Blackburn CC, Manley NR. Bmp4 and Noggin expression during early thymus and parathyroid organogenesis. Gene Expr Patterns. 2006; 6:794–9.
Article18. Cozzolino M, Lu Y, Sato T, Yang J, Suarez IG, Brancaccio D, et al. A critical role for enhanced TGF-alpha and EGFR expression in the initiation of parathyroid hyperplasia in experimental kidney disease. Am J Physiol Renal Physiol. 2005; 289:F1096–102.19. Kano M, Mizutani E, Homma S, Masaki H, Nakauchi H. Xenotransplantation and interspecies organogenesis: current status and issues. Front Endocrinol (Lausanne). 2022; 13:963282.
Article20. Chen J, Lansford R, Stewart V, Young F, Alt FW. RAG-2-deficient blastocyst complementation: an assay of gene function in lymphocyte development. Proc Natl Acad Sci U S A. 1993; 90:4528–32.
Article21. Kobayashi T, Yamaguchi T, Hamanaka S, Kato-Itoh M, Yamazaki Y, Ibata M, et al. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell. 2010; 142:787–99.
Article22. Yamaguchi T, Sato H, Kato-Itoh M, Goto T, Hara H, Sanbo M, et al. Interspecies organogenesis generates autologous functional islets. Nature. 2017; 542:191–6.
Article23. Chang AN, Liang Z, Dai HQ, Chapdelaine-Williams AM, Andrews N, Bronson RT, et al. Neural blastocyst complementation enables mouse forebrain organogenesis. Nature. 2018; 563:126–30.
Article24. Usui J, Kobayashi T, Yamaguchi T, Knisely AS, Nishinakamura R, Nakauchi H. Generation of kidney from pluripotent stem cells via blastocyst complementation. Am J Pathol. 2012; 180:2417–26.
Article25. Goto T, Hara H, Sanbo M, Masaki H, Sato H, Yamaguchi T, et al. Generation of pluripotent stem cell-derived mouse kidneys in Sall1-targeted anephric rats. Nat Commun. 2019; 10:451.
Article26. Kobayashi T, Goto T, Oikawa M, Sanbo M, Yoshida F, Terada R, et al. Blastocyst complementation using Prdm14-deficient rats enables efficient germline transmission and generation of functional mouse spermatids in rats. Nat Commun. 2021; 12:1328.
Article27. Isotani A, Hatayama H, Kaseda K, Ikawa M, Okabe M. Formation of a thymus from rat ES cells in xenogeneic nude mouse↔rat ES chimeras. Genes Cells. 2011; 16:397–405.
Article28. Hamanaka S, Umino A, Sato H, Hayama T, Yanagida A, Mizuno N, et al. Generation of vascular endothelial cells and hematopoietic cells by blastocyst complementation. Stem Cell Reports. 2018; 11:988–97.
Article29. Das S, Koyano-Nakagawa N, Gafni O, Maeng G, Singh BN, Rasmussen T, et al. Generation of human endothelium in pig embryos deficient in ETV2. Nat Biotechnol. 2020; 38:297–302.
Article30. Mori M, Furuhashi K, Danielsson JA, Hirata Y, Kakiuchi M, Lin CS, et al. Generation of functional lungs via conditional blastocyst complementation using pluripotent stem cells. Nat Med. 2019; 25:1691–8.
Article31. Kano M, Mizuno N, Sato H, Kimura T, Hirochika R, Iwasaki Y, et al. Functional calcium-responsive parathyroid glands generated using single-step blastocyst complementation. Proc Natl Acad Sci U S A. 2023; 120:e2216564120.
Article32. Akiyama Y, Hosoya T, Poole AM, Hotta Y. The gcm-motif: a novel DNA-binding motif conserved in Drosophila and mammals. Proc Natl Acad Sci U S A. 1996; 93:14912–6.33. Masaki H, Kato-Itoh M, Takahashi Y, Umino A, Sato H, Ito K, et al. Inhibition of apoptosis overcomes stage-related compatibility barriers to chimera formation in mouse embryos. Cell Stem Cell. 2016; 19:587–92.34. Nishimura T, Suchy FP, Bhadury J, Igarashi KJ, Charlesworth CT, Nakauchi H. Generation of functional organs using a cell-competitive niche in intra- and inter-species rodent chimeras. Cell Stem Cell. 2021; 28:141–9.
Article35. Tan T, Wu J, Si C, Dai S, Zhang Y, Sun N, et al. Chimeric contribution of human extended pluripotent stem cells to monkey embryos ex vivo. Cell. 2021; 184:2020–32.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Disease-specific pluripotent stem cells
- Induced Pluripotent Stem Cells: Next Generation Stem Cells to Clinical Applications
- Induced pluripotent stem cells and personalized medicine: current progress and future perspectives
- Inhibition of Class I Histone Deacetylase Enhances Self-Reprogramming of Spermatogonial Stem Cells into Pluripotent Stem Cells
- A Simple Method for Generating Cerebral Organoids from Human Pluripotent Stem Cells