J Korean Neurosurg Soc.
2024 Sep;67(5):510-520. 10.3340/jkns.2023.0189.
Neuro-Restorative Effect of Nimodipine and Calcitriol in 1-Methyl 4-Phenyl 1,2,3,6 Tetrahydropyridine-Induced Zebrafish Parkinson’s Disease Model
- Affiliations
-
- 1Department of Neurosurgery, Ansan Hospital, Korea University Medical Center, Korea University College of Medicine, Seoul, Korea
- 2Department of Biomedical Sciences, Korea University College of Medicine, Seoul, Korea
- KMID: 2558676
- DOI: http://doi.org/10.3340/jkns.2023.0189
Abstract
Objective
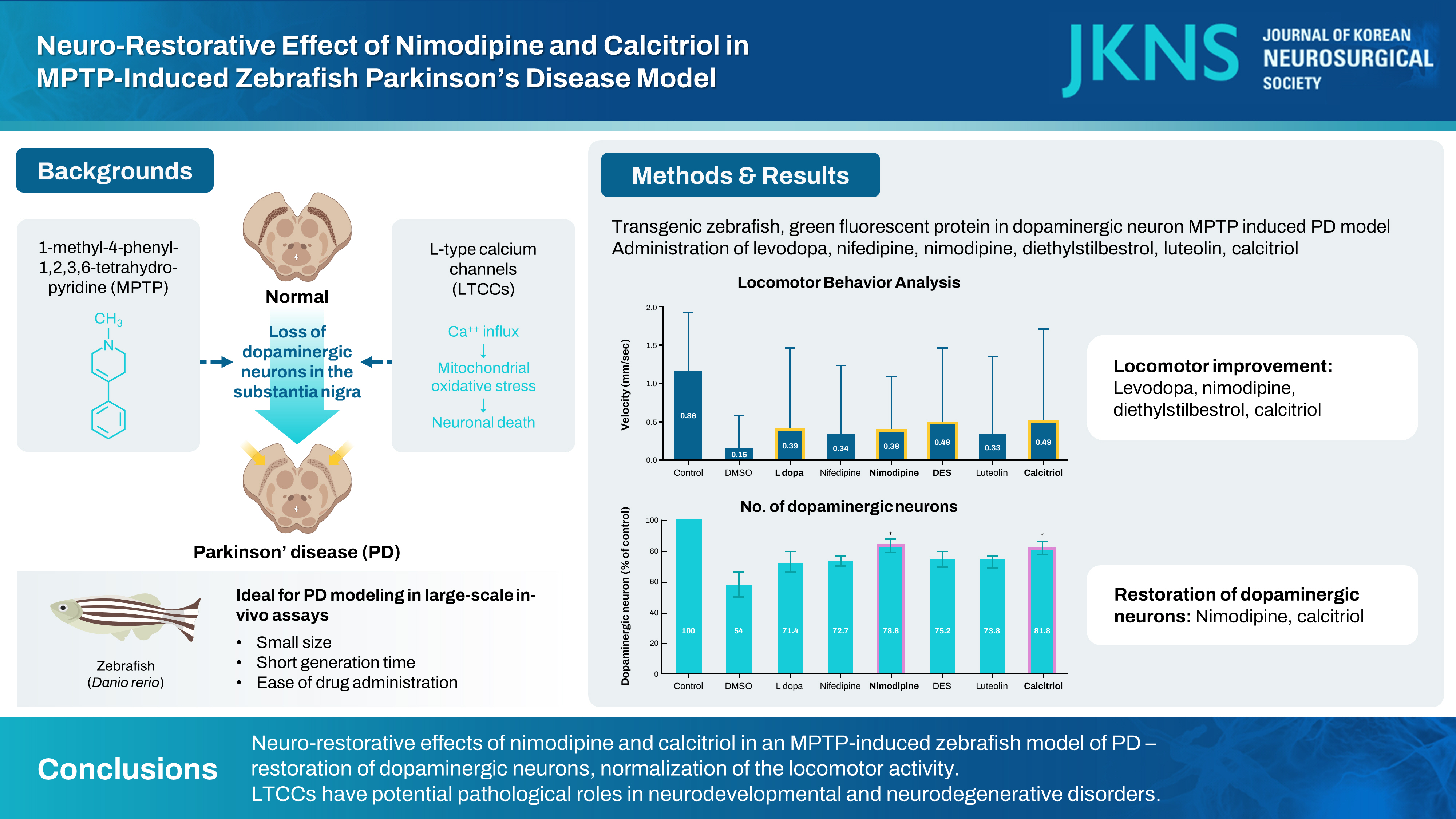
: Parkinson’s disease (PD) is one of the most prevalent neurodegenerative diseases, characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta. The treatment of PD aims to alleviate motor symptoms by replacing the reduced endogenous dopamine. Currently, there are no disease-modifying agents for the treatment of PD. Zebrafish (Danio rerio) have emerged as an effective tool for new drug discovery and screening in the age of translational research. The neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is known to cause a similar loss of dopaminergic neurons in the human midbrain, with corresponding Parkinsonian symptoms. L-type calcium channels (LTCCs) have been implicated in the generation of mitochondrial oxidative stress, which underlies the pathogenesis of PD. Therefore, we investigated the neuro-restorative effect of LTCC inhibition in an MPTP-induced zebrafish PD model and suggested a possible drug candidate that might modify the progression of PD.
Methods
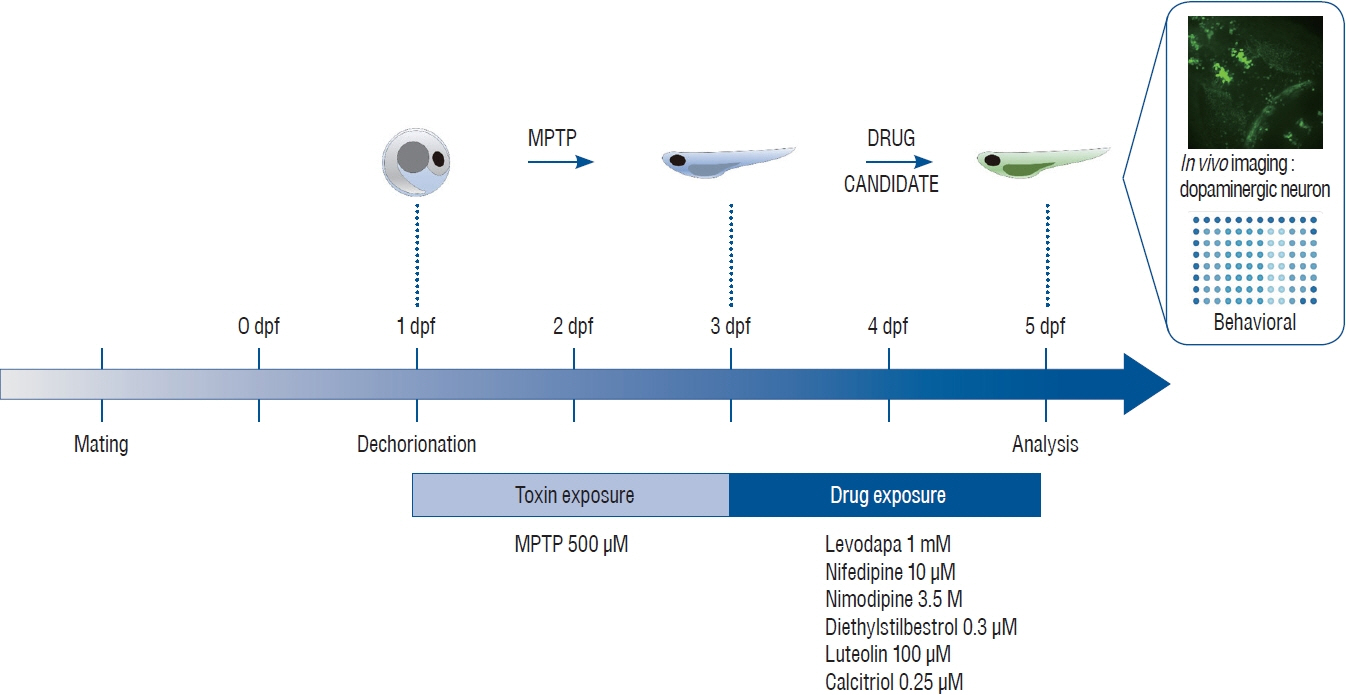
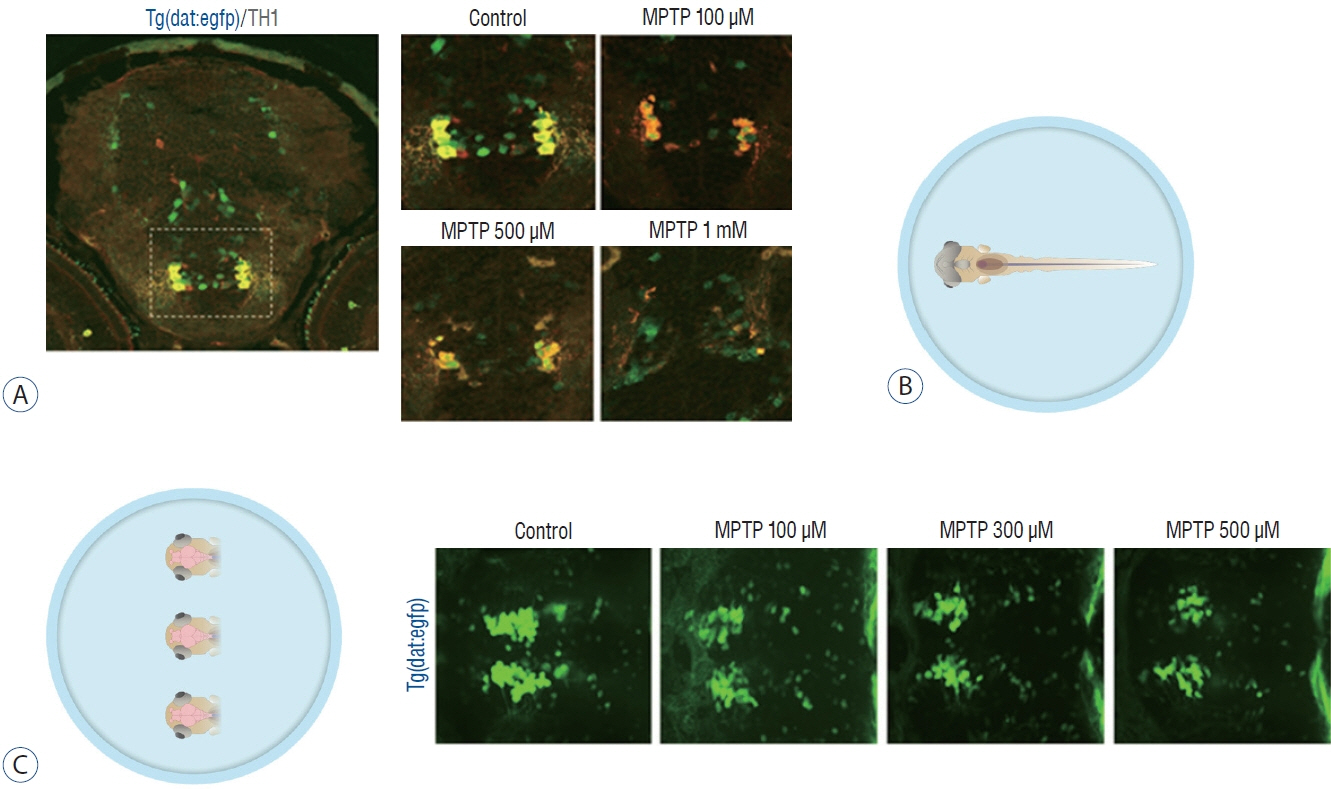
: All experiments were conducted using a line of transgenic zebrafish, Tg(dat:EGFP), in which green fluorescent protein (GFP) is expressed in dopaminergic neurons. The experimental groups were exposed to 500 μmol MPTP from 1 to 3 days post fertilization (dpf). The drug candidates : levodopa 1 mmol, nifedipine 10 μmol, nimodipine 3.5 μmol, diethylstilbestrol 0.3 μmol, luteolin 100 μmol, and cacitriol 0.25 μmol were exposed from 3 to 5 dpf. Locomotor activity was assessed by automated tracking and dopaminergic neurons were visualized in vivo by confocal microscopy.
Results
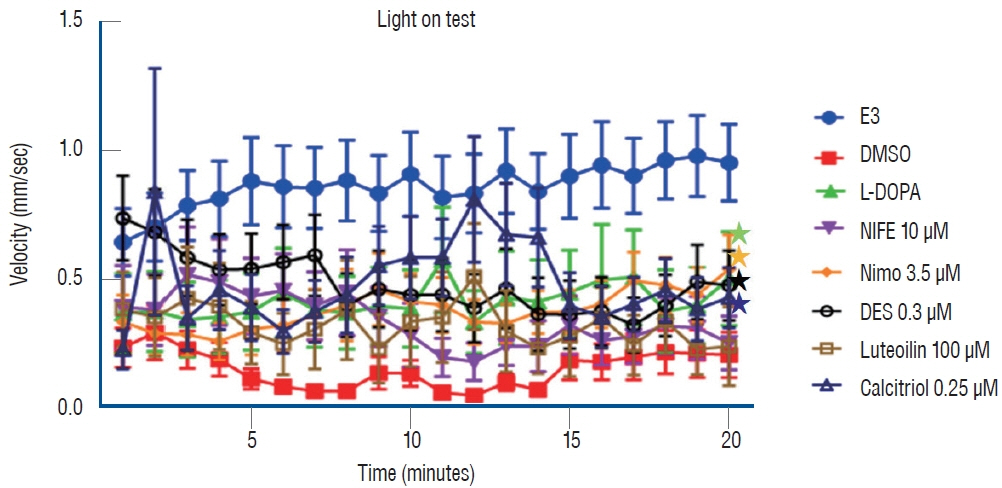
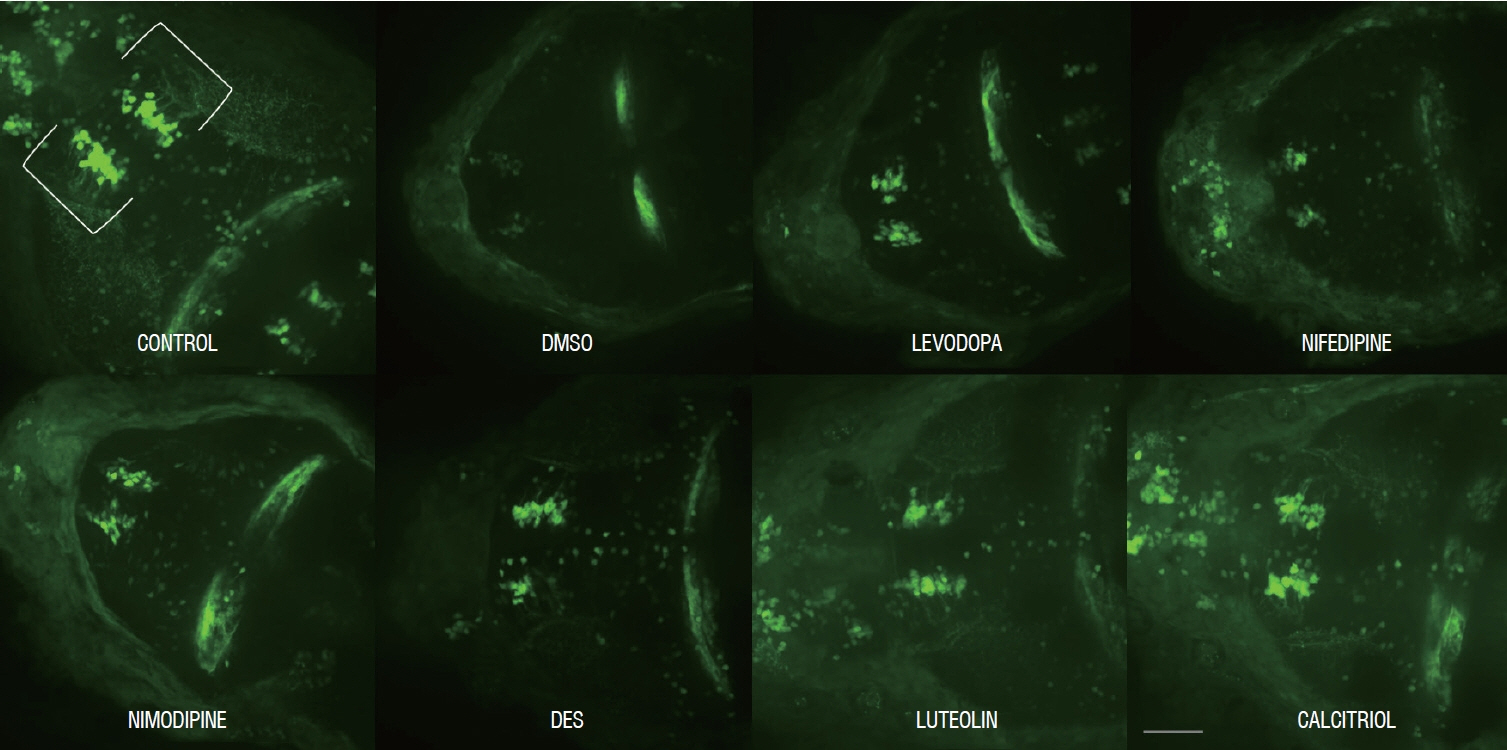
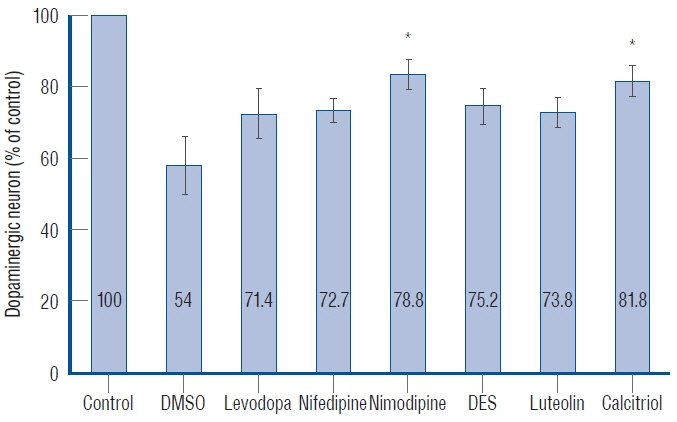
: Levodopa, nimodipine, diethylstilbestrol, and calcitriol had significant positive effects on the restoration of motor behavior, which was damaged by MPTP. Nimodipine and calcitriol have significant positive effects on the restoration of dopaminergic neurons, which were reduced by MPTP. Through locomotor analysis and dopaminergic neuron quantification, we identified the neuro-restorative effects of nimodipine and calcitriol in zebrafish MPTP-induced PD model.
Conclusion
: The present study identified the neuro-restorative effects of nimodipine and calcitriol in an MPTP-induced zebrafish model of PD. They restored dopaminergic neurons which were damaged due to the effects of MPTP and normalized the locomotor activity. LTCCs have potential pathological roles in neurodevelopmental and neurodegenerative disorders. Zebrafish are highly amenable to high-throughput drug screening and might, therefore, be a useful tool to work towards the identification of diseasemodifying treatment for PD. Further studies including zebrafish genetic models to elucidate the mechanism of action of the diseasemodifying candidate by investigating Ca2+ influx and mitochondrial function in dopaminergic neurons, are needed to reveal the pathogenesis of PD and develop disease-modifying treatments for PD.
Keyword
Figure
Cited by 1 articles
-
Editors’ Pick in September 2024
Hee-Jin Yang
J Korean Neurosurg Soc. 2024;67(5):487-488. doi: 10.3340/jkns.2024.0153.
Reference
-
References
1. Biglan KM, Oakes D, Lang AE, Hauser RA, Hodgeman K, Greco B, et al. A novel design of a phase III trial of isradipine in early Parkinson disease (STEADY-PD III). Ann Clin Transl Neurol. 4:360–368. 2017.2. Bretaud S, Lee S, Guo S. Sensitivity of zebrafish to environmental toxins implicated in Parkinson’s disease. Neurotoxicol Teratol. 26:857–864. 2004.
Article3. Carlson AP, Hänggi D, Macdonald RL, Shuttleworth CW. Nimodipine reappraised: an old drug with a future. Curr Neuropharmacol. 18:65–82. 2020.
Article4. Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, et al. ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature. 447:1081–1086. 2007.
Article5. Chang CC, Cao S, Kang S, Kai L, Tian X, Pandey P, et al. Antagonism of 4-substituted 1,4-dihydropyridine-3,5-dicarboxylates toward voltagedependent L-type Ca2+ channels Ca V 1.3 and Ca V 1.2. Bioorg Med Chem. 18:3147–3158. 2010.
Article6. Cui X, Gooch H, Petty A, McGrath JJ, Eyles D. Vitamin D and the brain: genomic and non-genomic actions. Mol Cell Endocrinol. 453:131–143. 2017.
Article7. DiPalma JR. Nimodipine in subarachnoid hemorrhage. Am Fam Physician. 40:143–145. 1989.8. Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 34:47–64. 2013.
Article9. Eyles DW, Feron F, Cui X, Kesby JP, Harms LH, Ko P, et al. Developmental vitamin D deficiency causes abnormal brain development. Psychoneuroendocrinology 34 Suppl. 1:S247–S257. 2009.
Article10. Flinn L, Bretaud S, Lo C, Ingham PW, Bandmann O. Zebrafish as a new animal model for movement disorders. J Neurochem. 106:1991–1997. 2008.
Article11. Gooch H, Cui X, Anggono V, Trzaskowski M, Tan MC, Eyles DW, et al. 1,25-Dihydroxyvitamin D modulates L-type voltage-gated calcium channels in a subset of neurons in the developing mouse prefrontal cortex. Transl Psychiatry. 9:281. 2019.
Article12. Grünblatt E, Mandel S, Youdim MB. MPTP and 6-hydroxydopamineinduced neurodegeneration as models for Parkinson’s disease: neuroprotective strategies. J Neurol. 247 Suppl 2:II95–II102. 2000.13. Guzman JN, Sanchez-Padilla J, Wokosin D, Kondapalli J, Ilijic E, Schumacker PT, et al. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature. 468:696–700. 2010.
Article14. Han Y, Chen A, Umansky KB, Oonk KA, Choi WY, Dickson AL, et al. Vitamin D stimulates cardiomyocyte proliferation and controls organ size and regeneration in zebrafish. Dev Cell. 48:853–863.e5. 2019.
Article15. Holzschuh J, Ryu S, Aberger F, Driever W. Dopamine transporter expression distinguishes dopaminergic neurons from other catecholaminergic neurons in the developing zebrafish embryo. Mech Dev. 101:237–243. 2001.
Article16. Ilijic E, Guzman JN, Surmeier DJ. The L-type channel antagonist isradipine is neuroprotective in a mouse model of Parkinson’s disease. Neurobiol Dis. 43:364–371. 2011.17. Jeong JY, Kwon HB, Ahn JC, Kang D, Kwon SH, Park JA, et al. Functional and developmental analysis of the blood-brain barrier in zebrafish. Brain Res Bull. 75:619–628. 2008.
Article18. Kalia LV, Lang AE. Parkinson’s disease. Lancet. 386:896–912. 2015.
Article19. Kang S, Cooper G, Dunne SF, Dusel B, Luan CH, Surmeier DJ, et al. CaV1.3-selective L-type calcium channel antagonists as potential new therapeutics for Parkinson’s disease. Nat Commun. 3:1146. 2012.
Article20. Kang S, Cooper G, Dunne SF, Luan CH, James Surmeier D, Silverman RB. Antagonism of L-type Ca2+ channels CaV1.3 and CaV1.2 by 1,4-dihydropyrimidines and 4H-pyrans as dihydropyridine mimics. Bioorg Med Chem. 21:4365–4373. 2013.
Article21. Kupsch A, Sautter J, Schwarz J, Riederer P, Gerlach M, Oertel WH. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in non-human primates is antagonized by pretreatment with nimodipine at the nigral, but not at the striatal level. Brain Res. 741:185–196. 1996.
Article22. Lam CS, Korzh V, Strahle U. Zebrafish embryos are susceptible to the dopaminergic neurotoxin MPTP. Eur J Neurosci. 21:1758–1762. 2005.
Article23. Lee KS, Huh S, Lee S, Wu Z, Kim AK, Kang HY, et al. Altered ER-mitochondria contact impacts mitochondria calcium homeostasis and contributes to neurodegeneration in vivo in disease models. Proc Natl Acad Sci U S A. 115:E8844–E8853. 2018.
Article24. Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 8:353–367. 2007.
Article25. Miller GW, Gainetdinov RR, Levey AI, Caron MG. Dopamine transport ers and neuronal injury. Trends Pharmacol Sci. 20:424–429. 1999.26. Nakajima T, Kitazawa T, Hamada E, Hazama H, Omata M, Kurachi Y. 17beta-estradiol inhibits the voltage-dependent L-type Ca2+ currents in aortic smooth muscle cells. Eur J Pharmacol. 294:625–635. 1995.
Article27. Nedergaard S, Flatman JA, Engberg I. Nifedipine- and omega-conotoxin-sensitive Ca2+ conductances in guinea-pig substantia nigra pars compacta neurones. J Physiol. 466:727–747. 1993.
Article28. Nicotra A, Parvez SH. Cell death induced by MPTP, a substrate for monoamine oxidase B. Toxicology. 153:157–166. 2000.
Article29. Oliveri AN, Glazer L, Mahapatra D, Kullman SW, Levin ED. Developmental exposure of zebrafish to vitamin D receptor acting drugs and environmental toxicants disrupts behavioral function. Neurotoxicol Teratol. 81:106902. 2020.
Article30. Ortner NJ, Striessnig J. L-type calcium channels as drug targets in CNS disorders. Channels (Austin). 10:7–13. 2016.
Article31. Panula P, Sallinen V, Sundvik M, Kolehmainen J, Torkko V, Tiittula A, et al. Modulatory neurotransmitter systems and behavior: towards zebrafish models of neurodegenerative diseases. Zebrafish. 3:235–247. 2006.
Article32. Parkinson Study Group. Phase II safety, tolerability, and dose selection study of isradipine as a potential disease-modifying intervention in early Parkinson’s disease (STEADY-PD). Mov Disord. 28:1823–1831. 2013.
Article33. Ramirez AD, Wong SK, Menniti FS. Pramipexole inhibits MPTP toxicity in mice by dopamine D3 receptor dependent and independent mechanisms. Eur J Pharmacol. 475:29–35. 2003.
Article34. Rink E, Wullimann MF. The teleostean (zebrafish) dopaminergic system ascending to the subpallium (striatum) is located in the basal diencephalon (posterior tuberculum). Brain Res. 889:316–330. 2001.
Article35. Sallinen V, Torkko V, Sundvik M, Reenilä I, Khrustalyov D, Kaslin J, et al. MPTP and MPP+ target specific aminergic cell populations in larval zebrafish. J Neurochem. 108:719–731. 2009.
Article36. Schoenrock SA, Tarantino LM. Developmental vitamin D deficiency and schizophrenia: the role of animal models. Genes Brain Behav. 15:45–61. 2016.37. Singh A, Verma P, Balaji G, Samantaray S, Mohanakumar KP. Nimodipine, an L-type calcium channel blocker attenuates mitochondrial dysfunctions to protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism in mice. Neurochem Int. 99:221–232. 2016.
Article38. Soman SK, Bazała M, Keatinge M, Bandmann O, Kuznicki J. Restriction of mitochondrial calcium overload by mcu inactivation renders a neuroprotective effect in zebrafish models of Parkinson’s disease. Biol Open. 8:bio044347. 2019.39. Sugishita K, Li F, Su Z, Barry WH. Anti-oxidant effects of estrogen reduce [Ca2+]i during metabolic inhibition. J Mol Cell Cardiol. 35:331–336. 2003.
Article40. Sulzer D. Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease. Trends Neurosci. 30:244–250. 2007.
Article41. Surmeier DJ, Schumacker PT, Guzman JD, Ilijic E, Yang B, Zampese E. Calcium and Parkinson’s disease. Biochem Biophys Res Commun. 483:1013–1019. 2017.
Article42. Swaminathan A, Basu M, Bekri A, Drapeau P, Kundu TK. The dietary flavonoid, luteolin, negatively affects neuronal differentiation. Front Mol Neurosci. 12:41. 2019.
Article43. Vaz RL, Outeiro TF, Ferreira JJ. Zebrafish as an animal model for drug discovery in Parkinson’s disease and other movement disorders: a systematic review. Front Neurol. 9:347. 2018.
Article44. Wagner M, Moritz A, Volk T. Interaction of gonadal steroids and the glucocorticoid corticosterone in the regulation of the L-type Ca(2+) current in rat left ventricular cardiomyocytes. Acta Physiol (Oxf). 202:629–640. 2011.
Article45. Xi Y, Ryan J, Noble S, Yu M, Yilbas AE, Ekker M. Impaired dopaminergic neuron development and locomotor function in zebrafish with loss of pink1 function. Eur J Neurosci. 31:623–633. 2010.
Article46. Xi Y, Yu M, Godoy R, Hatch G, Poitras L, Ekker M. Transgenic zebrafish expressing green fluorescent protein in dopaminergic neurons of the ventral diencephalon. Dev Dyn. 240:2539–2547. 2011.
Article47. Xie J, Farage E, Sugimoto M, Anand-Apte B. A novel transgenic zebrafish model for blood-brain and blood-retinal barrier development. BMC Dev Biol. 10:76. 2010.
Article48. Yagami T, Ueda K, Sakaeda T, Itoh N, Sakaguchi G, Okamura N, et al. Protective effects of a selective L-type voltage-sensitive calcium channel blocker, S-312-d, on neuronal cell death. Biochem Pharmacol. 67:1153–1165. 2004.
Article49. Yang M, Zhou Y, Wan LL, Ye JZ, Lu HL, Huang X, et al. Luteolin suppresses colonic smooth muscle motility via inhibiting L-type calcium channel currents in mice. Gen Physiol Biophys. 39:49–58. 2020.
Article50. Zhang ZJ, Cheang LC, Wang MW, Lee SM. Quercetin exerts a neuroprotective effect through inhibition of the iNOS/NO system and proinflammation gene expression in PC12 cells and in zebrafish. Int J Mol Med. 27:195–203. 2011.
Article51. Zhang ZJ, Cheang LC, Wang MW, Li GH, Chu IK, Lin ZX, et al. Ethanolic extract of fructus Alpinia oxyphylla protects against 6-hydroxydopamine-induced damage of PC12 cells in vitro and dopaminergic neurons in zebrafish. Cell Mol Neurobiol. 32:27–40. 2012.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Nicotine on MPTP-induced Parkinson's Disease Animal Model
- The Role of Axonopathy in Parkinson's Disease
- 1alpha,25-Dihydroxyvitamin D3 Protects Dopaminergic Neurons in Rodent Models of Parkinson's Disease through Inhibition of Microglial Activation
- Behavioral and Histochemical Changes in MPTP-treated C57BL/6 Mice: A Model for Parkinson's Disease
- The Effect of Ginseng Saponin on the Dopaminergic Neurons in the Parkinson's Disease Model in Mice