Int J Stem Cells.
2024 Aug;17(3):309-318. 10.15283/ijsc22200.
Human Endometrium Derived Mesenchymal Stem Cells with Aberrant NOD1 Expression Are Associated with Ectopic Endometrial Lesion Formation
- Affiliations
-
- 1Department of Gynecology and Obstetrics, Henan Provincial People’s Hospital, Zhengzhou University People’s Hospital, Henan University People’s Hospital, Zhengzhou, China
- 2Department of Gynecologic Oncology, Xinxiang Key Laboratory of Gynecological Endocrinology Diagnosis and Treatment, Xinxiang Central Hospital, The Fourth Affiliated Hospital of Xinxiang Medical College, Xinxiang, China
- 3Laboratory of Cell and Molecular Biology & State Key Laboratory of Molecular Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
- 4Department of Gynecology and Obstetrics, Women & Infants Hospital of Zhengzhou, Zhengzhou, China
- 5Department of Gynecologic Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
- KMID: 2558608
- DOI: http://doi.org/10.15283/ijsc22200
Abstract
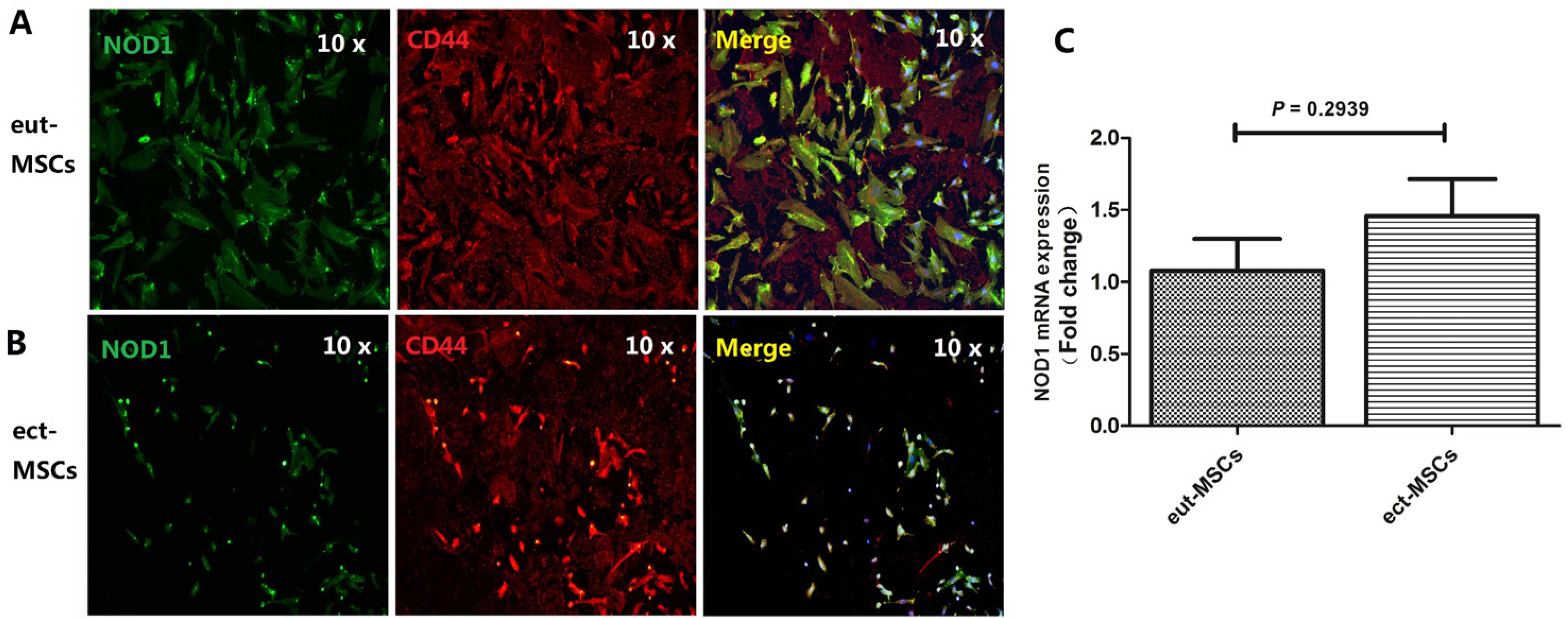
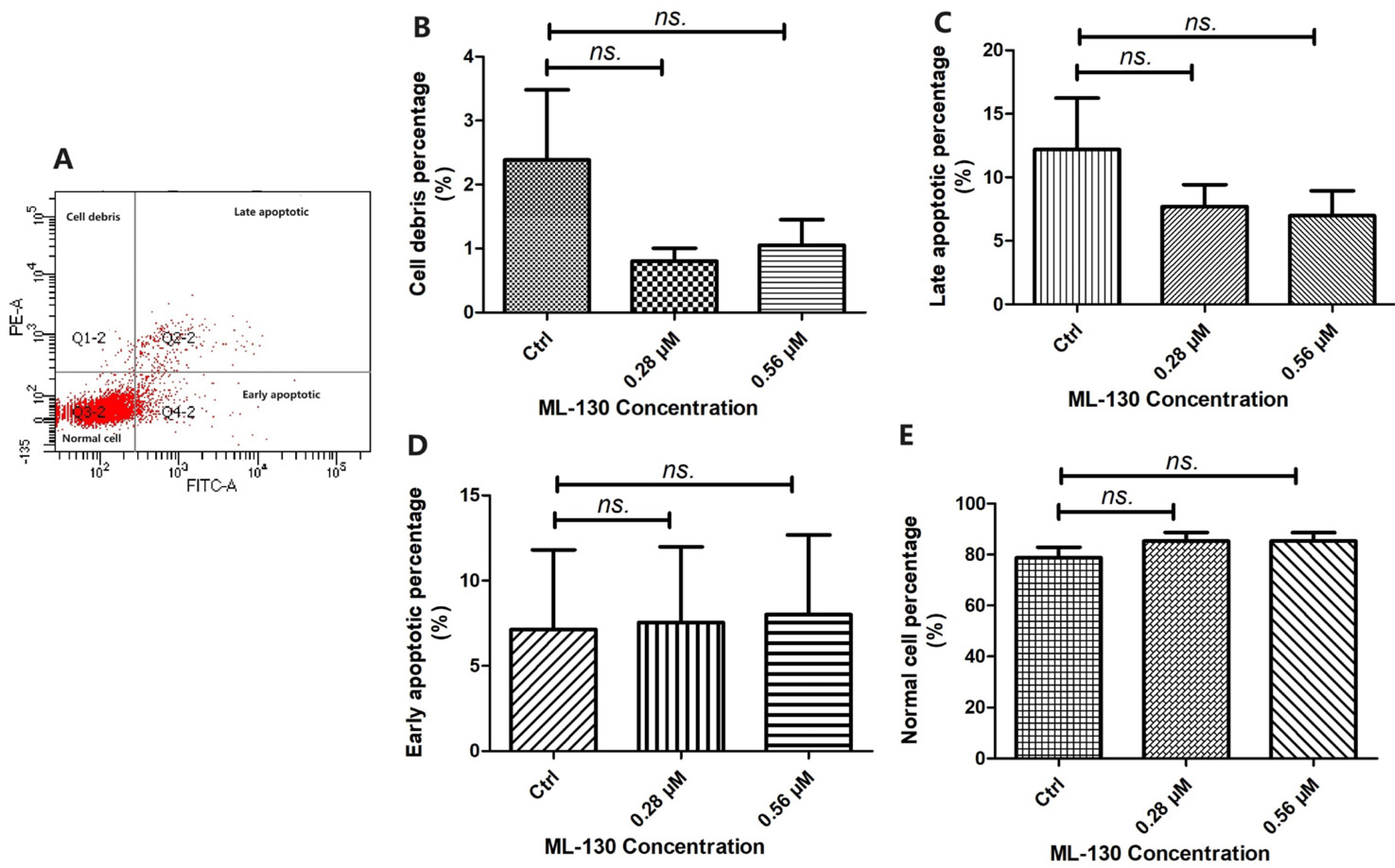
- Nucleotide-binding oligomerization domain 1 (NOD1), a cytosolic pattern recognition receptor protein, plays a crucial role in innate immune responses. However, the functional expression of NOD1 in mesenchymal stem cells (MSCs) derived from endometriosis remains unclear. The aim of this study was to explore the functions of NOD1 in ectopic endometrial lesions. Tissues and MSCs were isolated from both normal endometrium and endometriosis. Immunohistochemistry and real time quantitative polymerase chain reaction (RT-qPCR) were used to determine the expression of NOD1 in the tissues/MSCs. Quantification of various cytokines was performed using RT-qPCR and enzyme-linked immunosorbent assay. To confirm the proliferation, invasion/migration, and apoptotic viabilities of the samples, Cell Counting Kit-8, clonogenic formation, transwell assays, and apoptotic experiments were conducted. Higher levels of NOD1 expression were detected in the ectopic-MSCs obtained from endometriosis compared to those from the endometrium. The expression of interleukin-8 was higher in the ectopic-MSCs than in the eutopic-MSCs. Pretreatment with NOD1 agonist significantly enhanced the proliferation and invasion/migration of eutopic-MSCs. Additionally, the NOD1 inhibitor ML-130 significantly reduced the proliferation, clone formation, invasion, and migration abilities of the ectopic-MSCs, having no effect on their apoptosis capacity. Our findings suggest that the expression of NOD1 in ectopic-MSCs may contribute to the progression of ectopic endometrial lesions.
Keyword
Figure
Reference
-
References
1. Cook CJ, Wiggin N, Fogg KC. 2024; Characterizing the extracellular matrix transcriptome of endometriosis. Reprod Sci. 31:413–429. DOI: 10.1007/s43032-023-01359-w. PMID: 37789126. PMCID: PMC10827821.2. Kao AP, Wang KH, Chang CC, et al. 2011; Comparative study of human eutopic and ectopic endometrial mesenchymal stem cells and the development of an in vivo endometriotic invasion model. Fertil Steril. 95:1308–1315.e1. DOI: 10.1016/j.fertnstert.2010.09.064. PMID: 21047634.3. Patel BG, Lenk EE, Lebovic DI, Shu Y, Yu J, Taylor RN. 2018; Pathogenesis of endometriosis: interaction between endocrine and inflammatory pathways. Best Pract Res Clin Obstet Gynaecol. 50:50–60. DOI: 10.1016/j.bpobgyn.2018.01.006. PMID: 29576469.4. Pei G, Dorhoi A. 2021; NOD-like receptors: guards of cellular homeostasis perturbation during infection. Int J Mol Sci. 22:6714. DOI: 10.3390/ijms22136714. PMID: 34201509. PMCID: PMC8268748.5. Zhang Y, Zhang Y, Li C, et al. 2019; NOD1 modulates decidual stromal cell function to maintain pregnancy in the early trimester. Cell Biochem Funct. 37:464–473. DOI: 10.1002/cbf.3417. PMID: 31396989.6. King AE, Horne AW, Hombach-Klonisch S, Mason JI, Critchley HO. 2009; Differential expression and regulation of nuclear oligomerization domain proteins NOD1 and NOD2 in human endometrium: a potential role in innate immune protection and menstruation. Mol Hum Reprod. 15:311–319. DOI: 10.1093/molehr/gap020. PMID: 19273470.7. Zhang Y, Yang C, Fu S, et al. 2014; Different expression of NOD2 in decidual stromal cells between normal and unexplained recurrent spontaneous abortion women during first trimester gestation. Int J Clin Exp Pathol. 7:8784–8790. PMID: 25674246. PMCID: PMC4313978.8. Diao R, Wei W, Zhao J, Tian F, Cai X, Duan YG. 2017; CCL19/CCR7 contributes to the pathogenesis of endometriosis via PI3K/Akt pathway by regulating the proliferation and invasion of ESCs. Am J Reprod Immunol. 78:e12744. DOI: 10.1111/aji.12744. PMID: 28856757.9. Zhu L, Wang Y, Zhu Y, Zhang W, Zhu J. 2018; Expression of NOD1 and downstream factors in placenta, fetal membrane and plasma from pregnancies with premature rupture of membranes and their significance. Int J Clin Exp Pathol. 11:5745–5754. PMID: 31949660. PMCID: PMC6963101.10. Zhang Y, Li N, Yuan G, et al. 2022; Upregulation of NOD1 and NOD2 contribute to cancer progression through the positive regulation of tumorigenicity and metastasis in human squamous cervical cancer. BMC Med. 20:55. DOI: 10.1186/s12916-022-02248-w. PMID: 35130902. PMCID: PMC8822783.11. Rahmawati NY, Ahsan F, Santoso B, et al. 2023; IL-8 and IL-12p70 are associated with pelvic pain among infertile women with endometriosis. Pain Med. 24:1262–1269. DOI: 10.1093/pm/pnad080. PMID: 37326977.12. Crispim PCA, Jammal MP, Murta EFC, Nomelini RS. 2021; Endometriosis: what is the influence of immune cells? Immunol Invest. 50:372–388. DOI: 10.1080/08820139.2020.1764577. PMID: 32408782.13. Ramírez-Pavez TN, Martínez-Esparza M, Ruiz-Alcaraz AJ, Marín-Sánchez P, Machado-Linde F, García-Peñarrubia P. 2021; The role of peritoneal macrophages in endometriosis. Int J Mol Sci. 22:10792. DOI: 10.3390/ijms221910792. PMID: 34639133. PMCID: PMC8509388.14. Guo B, Chen JH, Zhang JH, et al. 2023; Pattern-recognition receptors in endometriosis: a narrative review. Front Immu-nol. 14:1161606. DOI: 10.3389/fimmu.2023.1161606. PMID: 37033937. PMCID: PMC10076794.15. Buchholz KR, Stephens RS. 2008; The cytosolic pattern recognition receptor NOD1 induces inflammatory interleukin-8 during Chlamydia trachomatis infection. Infect Immun. 76:3150–3155. DOI: 10.1128/IAI.00104-08. PMID: 18426885. PMCID: PMC2446689.16. Navarro R, Delgado-Wicke P, Nuñez-Prado N, et al. 2016; Role of nucleotide-binding oligomerization domain 1 (NOD1) in pericyte-mediated vascular inflammation. J Cell Mol Med. 20:980–986. DOI: 10.1111/jcmm.12804. PMID: 26915562. PMCID: PMC4831361.17. Luddi A, Marrocco C, Governini L, et al. 2020; Expression of matrix metalloproteinases and their inhibitors in endometrium: high levels in endometriotic lesions. Int J Mol Sci. 21:2840. DOI: 10.3390/ijms21082840. PMID: 32325785. PMCID: PMC7215833.18. Yang H, Liu J, Fan Y, et al. 2016; Associations between various possible promoter polymorphisms of MMPs genes and endometriosis risk: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 205:174–188. DOI: 10.1016/j.ejogrb.2016.08.015. PMID: 27620811.19. Muharam R, Rahmala Febri R, Mutia K, et al. 2023; Down-regulation of miR-93 negatively correlates with overexpression of VEGFA and MMP3 in endometriosis: a cross-sectional study. Int J Fertil Steril. 17:28–33. DOI: 10.22074/ijfs.2022.543884.1233. PMID: 36617199. PMCID: PMC9807893.20. Bruner-Tran KL, Eisenberg E, Yeaman GR, Anderson TA, McBean J, Osteen KG. 2002; Steroid and cytokine regulation of matrix metalloproteinase expression in endometriosis and the establishment of experimental endometriosis in nude mice. J Clin Endocrinol Metab. 87:4782–4791. DOI: 10.1210/jc.2002-020418. PMID: 12364474.21. Baranov VS, Ivaschenko TE, Liehr T, Yarmolinskaya MI. 2015; Systems genetics view of endometriosis: a common complex disorder. Eur J Obstet Gynecol Reprod Biol. 185:59–65. DOI: 10.1016/j.ejogrb.2014.11.036. PMID: 25528731.22. Delbandi AA, Mahmoudi M, Shervin A, Heidari S, Kola-hdouz-Mohammadi R, Zarnani AH. 2020; Evaluation of apoptosis and angiogenesis in ectopic and eutopic stromal cells of patients with endometriosis compared to non-endometriotic controls. BMC Womens Health. 20:3. DOI: 10.1186/s12905-019-0865-4. PMID: 31906916. PMCID: PMC6945780.23. Kasimsetty SG, Hawkes A, Barekatain K, Soo E, Welch AK, McKay DB. 2020; TLR2 and NODs1 and 2 cooperate in inflammatory responses associated with renal ischemia reperfusion injury. Transpl Immunol. 58:101260. DOI: 10.1016/j.trim.2019.101260. PMID: 31760144. PMCID: PMC7041897.24. Liu H, Zhang W, Wang L, et al. 2019; GLI1 is increased in ovarian endometriosis and regulates migration, invasion and proliferation of human endometrial stromal cells in endo-metriosis. Ann Transl Med. 7:663. DOI: 10.21037/atm.2019.10.76. PMID: 31930064. PMCID: PMC6944576.25. Burlev VA, Pavlovich SV, Il’yasova NA. 2006; Apoptosis and proliferative activity in endometrium during peritoneal endo-metriosis. Bull Exp Biol Med. 141:204–217. DOI: 10.1007/s10517-006-0128-x. PMID: 16984097.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bone marrow-derived stem cells contribute to regeneration of the endometrium
- Extracellular Vesicles Derived from Mesenchymal Stem Cells as Cell-Free Therapy for Intrauterine Adhesion
- Very Small Putative Stem Cells Detected in Human Endometrium
- Immunohistochemical Analysis of CD44s and CD44v6 in Endometriosis and Adenomyosis: Comparison with normal, hyperplastic, and malignant endometrium
- Evaluating the effect of conditioned medium from endometrial stem cells on endometriosis-derived endometrial stem cells