Anat Cell Biol.
2022 Mar;55(1):100-108. 10.5115/acb.21.169.
Evaluating the effect of conditioned medium from endometrial stem cells on endometriosis-derived endometrial stem cells
- Affiliations
-
- 1Department of Mesenchymal Stem Cells, The Academic Centre for Education, Culture and Research, Qom, Iran
- 2Department of Reproductive Biology, The Academic Centre for Education, Culture and Research, Qom, Iran
- KMID: 2527674
- DOI: http://doi.org/10.5115/acb.21.169
Abstract
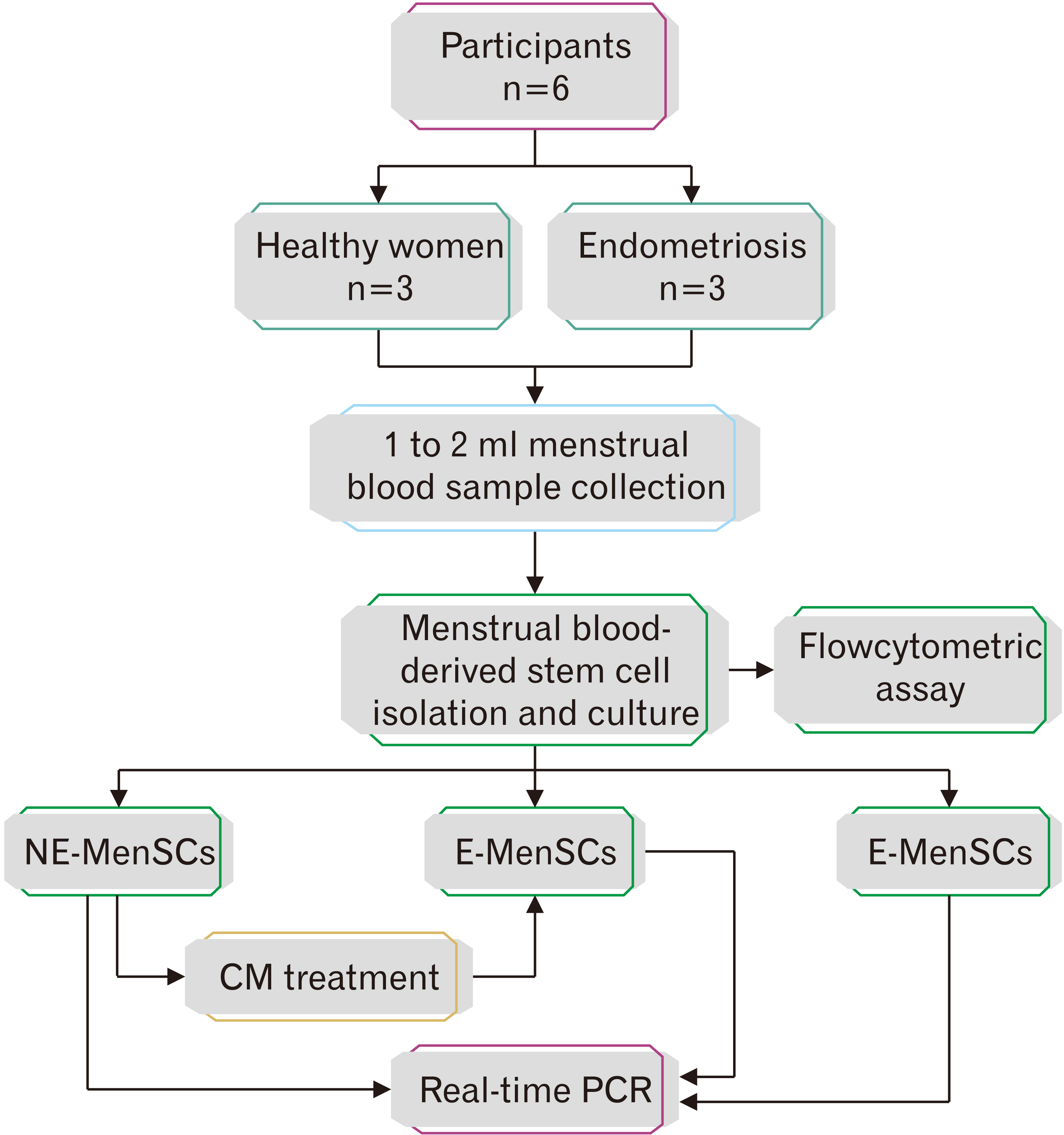
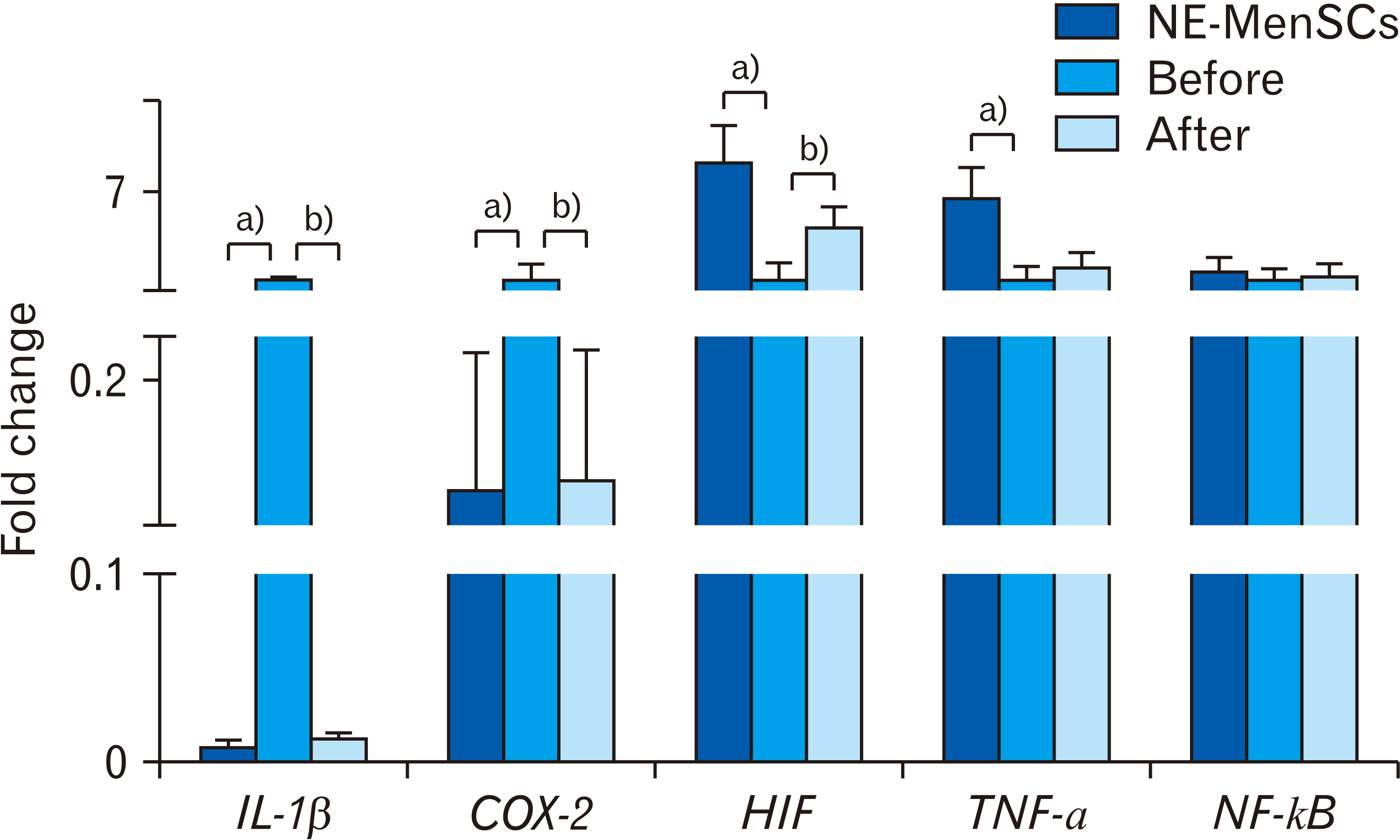
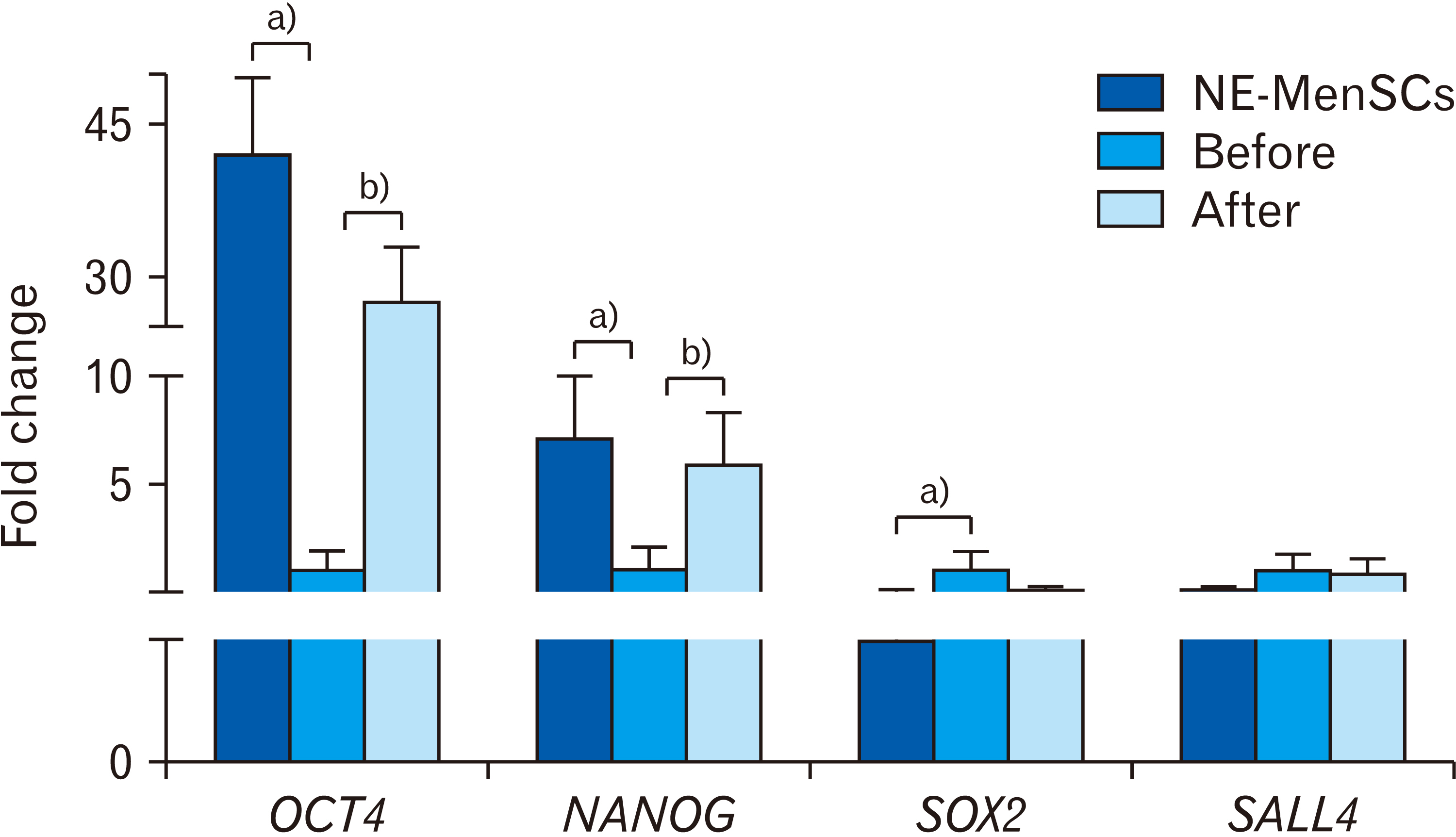
- Endometriosis is a common, benign gynecological disease which is determined as an overspreading of endometrial tissue in exterior region of the uterine cavity. Evidence suggests that retrograde menstrual blood which contains mesenchymal stem cells with differential gene expression compared to healthy women may play a role in endometriosis creation. We aimed to identify whether the conditioned medium (CM) from menstrual blood-derived mesenchymal stem cells (MenSCs) of healthy women can affect the expression level of inf lammatory and stemness genes of MenSCs from endometriosis women. Endometriosis-derived MenSCs (E-MenSCs) were treated with CM derived from healthy women’s MenSCs (non-endometriosis derived MenSCs [NE-MenSCs]). Some CD markers were analyzed by flow cytometer before and after treatment compared with NE-MenSCs, and the expression level of inflammatory and stemness genes was evaluated by real-time PCR. E-MenSCs show different morphology in vitro culture in comparison with NE-MenSCs, which were changed in the presence of CM, into a morphology more similar to normal cells and showed significant decrease expression of CD10 after CM treatment. In our results, the interleukin-1, cyclooxygenase-2, and hypoxia-inducible factor 1α as inflamaturay genes and octamer-binding transcription factor 4, NANOG, and sex determining region Y-box 2 as stemness genes showed significantly different expression level in E-MenSCs after treating with CM. Our study indicates that the expression level of some inflammatory- and stemness-related genes which have differential expression in E-MenSCs compared with NEMenSCs, could be changed to normal status by using CM derived from NE-MenSCs.
Figure
Reference
-
References
1. Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. 2018; Endometriosis. Nat Rev Dis Primers. 4:9. DOI: 10.1038/s41572-018-0008-5. PMID: 30026507.
Article2. Chen L, Qu J, Xiang C. 2019; The multi-functional roles of menstrual blood-derived stem cells in regenerative medicine. Stem Cell Res Ther. 10:1. DOI: 10.1186/s13287-018-1105-9. PMID: 30606242. PMCID: PMC6318883.
Article3. Bellelis P, Podgaec S, Abrão MS. 2011; Environmental factors and endometriosis. Rev Assoc Med Bras (1992). 57:448–52. DOI: 10.1016/S0104-4230(11)70093-8.
Article4. Persoons E, De Clercq K, Van den Eynde C, Pinto SJPC, Luyten K, Van Bree R, Tomassetti C, Voets T, Vriens J. 2020; Mimicking Sampson's retrograde menstrual theory in rats: a new rat model for ongoing endometriosis-associated pain. Int J Mol Sci. 21:2326. DOI: 10.3390/ijms21072326. PMID: 32230898. PMCID: PMC7177935.
Article5. Lv H, Hu Y, Cui Z, Jia H. 2018; Human menstrual blood: a renewable and sustainable source of stem cells for regenerative medicine. Stem Cell Res Ther. 9:325. DOI: 10.1186/s13287-018-1067-y. PMID: 30463587. PMCID: PMC6249727.
Article6. Bozorgmehr M, Gurung S, Darzi S, Nikoo S, Kazemnejad S, Zarnani AH, Gargett CE. 2020; Endometrial and menstrual blood mesenchymal stem/stromal cells: biological properties and clinical application. Front Cell Dev Biol. 8:497. DOI: 10.3389/fcell.2020.00497. PMID: 32742977. PMCID: PMC7364758.
Article7. Chang JH, Au HK, Lee WC, Chi CC, Ling TY, Wang LM, Kao SH, Huang YH, Tzeng CR. 2013; Expression of the pluripotent transcription factor OCT4 promotes cell migration in endometriosis. Fertil Steril. 99:1332–9.e5. DOI: 10.1016/j.fertnstert.2012.11.033. PMID: 23290742.
Article8. Deldar Y, Zarghami F, Pilehvar-Soltanahmadi Y, Dadashpour M, Zarghami N. 2017; Antioxidant effects of chrysin-loaded electrospun nanofibrous mats on proliferation and stemness preservation of human adipose-derived stem cells. Cell Tissue Bank. 18:475–87. DOI: 10.1007/s10561-017-9654-1. PMID: 28808812.
Article9. Fakih H, Baggett B, Holtz G, Tsang KY, Lee JC, Williamson HO. 1987; Interleukin-1: a possible role in the infertility associated with endometriosis. Fertil Steril. 47:213–7. DOI: 10.1016/S0015-0282(16)49993-0.
Article10. Banu SK, Lee J, Speights VO Jr, Starzinski-Powitz A, Arosh JA. 2008; Cyclooxygenase-2 regulates survival, migration, and invasion of human endometriotic cells through multiple mechanisms. Endocrinology. 149:1180–9. DOI: 10.1210/en.2007-1168. PMID: 18039779.
Article11. Kyama CM, Overbergh L, Debrock S, Valckx D, Vander Perre S, Meuleman C, Mihalyi A, Mwenda JM, Mathieu C, D'Hooghe TM. 2006; Increased peritoneal and endometrial gene expression of biologically relevant cytokines and growth factors during the menstrual phase in women with endometriosis. Fertil Steril. 85:1667–75. DOI: 10.1016/j.fertnstert.2005.11.060. PMID: 16759923.
Article12. Wu MH, Hsiao KY, Tsai SJ. 2015; Endometriosis and possible inflammation markers. Gynecol Minim Invasive Ther. 4:61–7. DOI: 10.1016/j.gmit.2015.05.001.
Article13. Proestling K, Birner P, Balendran S, Nirtl N, Marton E, Yerlikaya G, Kuessel L, Reischer T, Wenzl R, Streubel B, Husslein H. 2016; Enhanced expression of the stemness-related factors OCT4, SOX15 and TWIST1 in ectopic endometrium of endometriosis patients. Reprod Biol Endocrinol. 14:81. DOI: 10.1186/s12958-016-0215-4. PMID: 27881125. PMCID: PMC5122168.
Article14. Song Y, Xiao L, Fu J, Huang W, Wang Q, Zhang X, Yang S. 2014; Increased expression of the pluripotency markers sex-determining region Y-box 2 and Nanog homeobox in ovarian endometriosis. Reprod Biol Endocrinol. 12:42. DOI: 10.1186/1477-7827-12-42. PMID: 24884521. PMCID: PMC4031377.
Article15. Park JH, Daheron L, Kantarci S, Lee BS, Teixeira JM. 2011; Human endometrial cells express elevated levels of pluripotent factors and are more amenable to reprogramming into induced pluripotent stem cells. Endocrinology. 152:1080–9. DOI: 10.1210/en.2010-1072. PMID: 21209016. PMCID: PMC3198966.
Article16. Siu MK, Wong ES, Kong DS, Chan HY, Jiang L, Wong OG, Lam EW, Chan KK, Ngan HY, Le XF, Cheung AN. 2013; Stem cell transcription factor NANOG controls cell migration and invasion via dysregulation of E-cadherin and FoxJ1 and contributes to adverse clinical outcome in ovarian cancers. Oncogene. 32:3500–9. DOI: 10.1038/onc.2012.363. PMID: 22945654.
Article17. Zhang W, Sui Y, Ni J, Yang T. 2016; Insights into the Nanog gene: a propeller for stemness in primitive stem cells. Int J Biol Sci. 12:1372–81. DOI: 10.7150/ijbs.16349. PMID: 27877089. PMCID: PMC5118783.18. Forghanifard MM, Moghbeli M, Raeisossadati R, Tavassoli A, Mallak AJ, Boroumand-Noughabi S, Abbaszadegan MR. 2013; Role of SALL4 in the progression and metastasis of colorectal cancer. J Biomed Sci. 20:6. DOI: 10.1186/1423-0127-20-6. PMID: 23363002. PMCID: PMC3599462.
Article19. Ardalan Khales S, Abbaszadegan MR, Abdollahi A, Raeisossadati R, Tousi MF, Forghanifard MM. 2015; SALL4 as a new biomarker for early colorectal cancers. J Cancer Res Clin Oncol. 141:229–35. DOI: 10.1007/s00432-014-1808-y. PMID: 25156818.
Article20. Joseph A, Baiju I, Bhat IA, Pandey S, Bharti M, Verma M, Pratap Singh A, Ansari MM, Chandra V, Saikumar G, Amarpal , Taru Sharma G. 2020; Mesenchymal stem cell-conditioned media: a novel alternative of stem cell therapy for quality wound healing. J Cell Physiol. 235:5555–69. DOI: 10.1002/jcp.29486. PMID: 31960454.
Article21. Noverina R, Widowati W, Ayuningtyas W, Kurniawan D, Afifah E, Laksmitawati DR, Rinendyaputri R, Rilianawati R, Faried A, Bachtiar I, Wirakusumah FF. 2019; Growth factors profile in conditioned medium human adipose tissue-derived mesenchymal stem cells (CM-hATMSCs). Clin Nutr Exp. 24:34–44. DOI: 10.1016/j.yclnex.2019.01.002.
Article22. Sefati N, Norouzian M, Abbaszadeh HA, Abdollahifar MA, Amini A, Bagheri M, Aryan A, Fadaei Fathabady F. 2018; Effects of bone marrow mesenchymal stem cells-conditioned medium on tibial partial osteotomy model of fracture healing in hypothyroidism rats. Iran Biomed J. 22:90–8.23. Gauthaman K, Fong CY, Arularasu S, Subramanian A, Biswas A, Choolani M, Bongso A. 2013; Human Wharton's jelly stem cell conditioned medium and cell-free lysate inhibit human osteosarcoma and mammary carcinoma cell growth in vitro and in xenograft mice. J Cell Biochem. 114:366–77. DOI: 10.1002/jcb.24367. PMID: 22930595.24. Gauthaman K, Fong CY, Suganya CA, Subramanian A, Biswas A, Choolani M, Bongso A. 2012; Extra-embryonic human Wharton's jelly stem cells do not induce tumorigenesis, unlike human embryonic stem cells. Reprod Biomed Online. 24:235–46. DOI: 10.1016/j.rbmo.2011.10.007. PMID: 22196893.
Article25. Nikoo S, Ebtekar M, Jeddi-Tehrani M, Shervin A, Bozorgmehr M, Vafaei S, Kazemnejad S, Zarnani AH. 2014; Menstrual blood-derived stromal stem cells from women with and without endometriosis reveal different phenotypic and functional characteristics. Mol Hum Reprod. 20:905–18. DOI: 10.1093/molehr/gau044. PMID: 24939730.
Article26. Chalpe A, Law C, Dumdie J, Hansen K, Eyster K. 2015; TNFα and IL1β stimulate differential gene expression in endometrial stromal cells. Adv Biol Chem. 5:126–41. DOI: 10.4236/abc.2015.52010.27. Ota H, Igarashi S, Sasaki M, Tanaka T. 2001; Distribution of cyclooxygenase-2 in eutopic and ectopic endometrium in endometriosis and adenomyosis. Hum Reprod. 16:561–6. DOI: 10.1093/humrep/16.3.561. PMID: 11228229.
Article28. Matsuzaki S, Canis M, Pouly JL, Wattiez A, Okamura K, Mage G. 2004; Cyclooxygenase-2 expression in deep endometriosis and matched eutopic endometrium. Fertil Steril. 82:1309–15. DOI: 10.1016/j.fertnstert.2004.03.059. PMID: 15533352.
Article29. Nickoloff BJ, Qin JZ, Chaturvedi V, Denning MF, Bonish B, Miele L. 2002; Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-kappaB and PPARgamma. Cell Death Differ. 9:842–55. DOI: 10.1038/sj.cdd.4401036. PMID: 12107827.
Article30. Santamaria X, Massasa EE, Taylor HS. 2012; Migration of cells from experimental endometriosis to the uterine endometrium. Endocrinology. 153:5566–74. DOI: 10.1210/en.2012-1202. PMID: 22968642. PMCID: PMC3473215.
Article31. Liu L, Zhang J, Yang X, Fang C, Xu H, Xi X. 2015; SALL4 as an epithelial-mesenchymal transition and drug resistance inducer through the regulation of c-Myc in endometrial cancer. PLoS One. 10:e0138515. DOI: 10.1371/journal.pone.0138515. PMID: 26407074. PMCID: PMC4583418.
Article32. Lin SC, Li YH, Wu MH, Chang YF, Lee DK, Tsai SY, Tsai MJ, Tsai SJ. 2014; Suppression of COUP-TFII by proinflammatory cytokines contributes to the pathogenesis of endometriosis. J Clin Endocrinol Metab. 99:E427–37. DOI: 10.1210/jc.2013-3717. PMID: 24423359. PMCID: PMC5393480.
Article33. Götte M, Wolf M, Staebler A, Buchweitz O, Kiesel L, Schüring AN. 2011; Aberrant expression of the pluripotency marker SOX-2 in endometriosis. Fertil Steril. 95:338–41. DOI: 10.1016/j.fertnstert.2010.08.006. PMID: 20850729.
Article34. Zhu C, Yu J, Pan Q, Yang J, Hao G, Wang Y, Li L, Cao H. 2016; Hypoxia-inducible factor-2 alpha promotes the proliferation of human placenta-derived mesenchymal stem cells through the MAPK/ERK signaling pathway. Sci Rep. 6:35489. DOI: 10.1038/srep35489. PMID: 27765951. PMCID: PMC5073233.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bone marrow-derived stem cells contribute to regeneration of the endometrium
- Effect of Conditioned Medium from Human Umbilical Cord-Derived Mesenchymal Stromal Cells on Rejuvenation of Nucleus Pulposus Derived Stem/Progenitor Cells from Degenerated Intervertebral Disc
- Evaluating the effect of conditioned medium from mesenchymal stem cells on differentiation of rat spermatogonial stem cells
- Cultivation of the Isolated Bovine Endometrial Stromal Cells and the Effect of Interleukin - 2 on Its Proliferation
- Two Cases of Low-Grade Endometrial Stromal Sarcoma