J Korean Med Sci.
2024 Aug;39(32):e228. 10.3346/jkms.2024.39.e228.
COVID-19’s Radiologic, Functional, and Serologic Consequences at 6-Month and 18-Month Follow-up: A Prospective Cohort Study
- Affiliations
-
- 1Department of Radiology, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea
- 2Division of Infectious Diseases, Department of Internal Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea
- 3Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, GA, USA
- 4Translational Laboratory for Cardiothoracic Imaging and Artificial Intelligence, Emory University School of Medicine, Atlanta, GA, USA
- 5Division of Cardiothoracic Imaging, Department of Radiology, Emory University, Atlanta, GA, USA
- 6Medical Science Research Center, Korea University College of Medicine, Seoul, Korea
- 7Department of Radiology, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- 8Department of Radiology, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- KMID: 2558528
- DOI: http://doi.org/10.3346/jkms.2024.39.e228
Abstract
- Background
We evaluated the radiologic, pulmonary functional, and antibody statuses of coronavirus disease 2019 (COVID-19) patients 6 and 18 months after discharge, comparing changes in status and focusing on risk factors for residual computed tomography (CT) abnormalities.
Methods
This prospective cohort study was conducted on COVID-19 patients discharged between April 2020 and January 2021. Chest CT, pulmonary function testing (PFT), and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) immunoglobulin G (IgG) measurements were performed 6 and 18 months after discharge. We evaluated factors associated with residual CT abnormalities and the correlation between lesionvolume in CT (lesionvolume ), PFT, and IgG levels.
Results
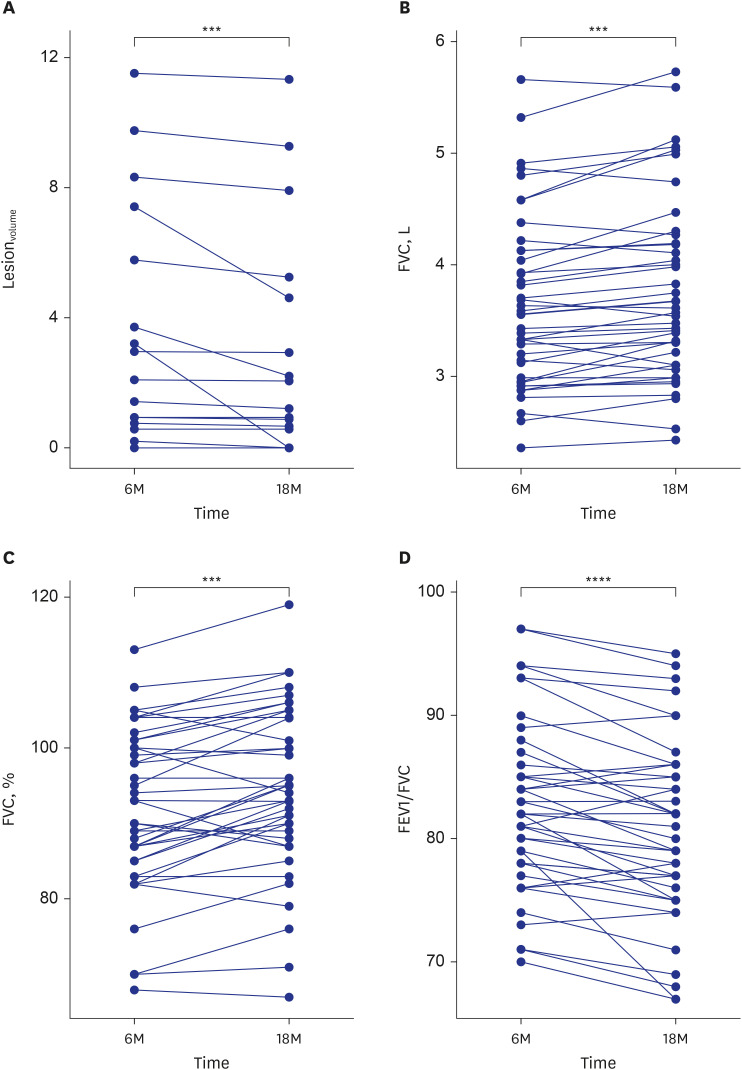
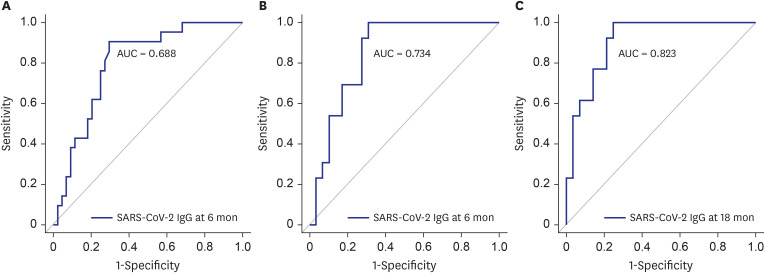
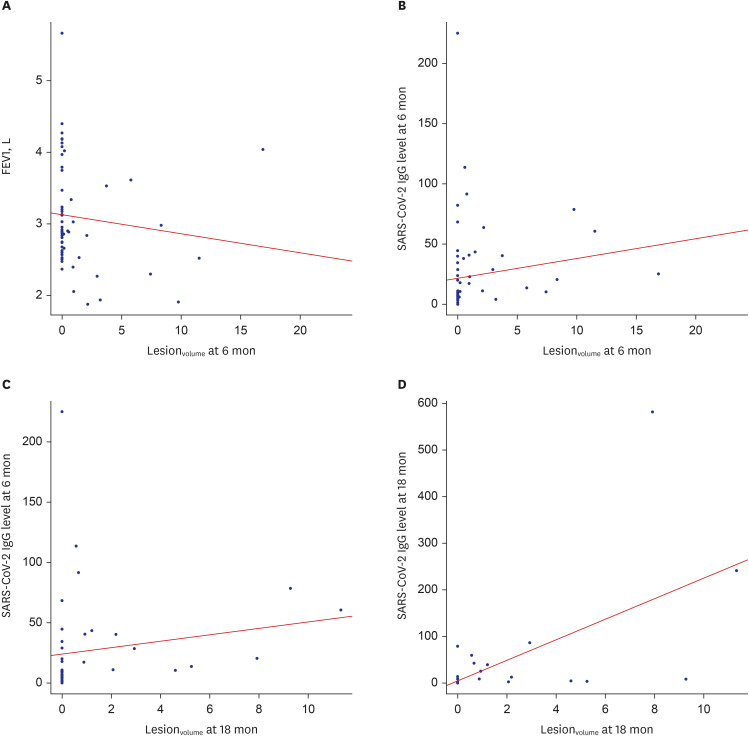
This study included 68 and 42 participants evaluated 6 and 18 months, respectively, after hospitalizations for COVID-19. CT abnormalities were noted in 22 participants (32.4%) at 6 months and 13 participants (31.0%) at 18 months. Lesionvolume was significantly lower at 18 months than 6 months (P < 0.001). Patients with CT abnormalities at 6 months showed lower forced expiratory volume in 1 second (FEV1) and FEV1/forced vital capacity (FVC), and patients with CT abnormalities at 18 months exhibited lower FVC. FVC significantly improved between 6 and 18 months of follow-up (all P < 0.0001). SARS-CoV-2 IgG levels were significantly higher in patients with CT abnormalities at 6 and 18 months (P < 0.001). At 18-month follow-up assessments, age was associated with CT abnormalities (odds ratio, 1.17; 95% confidence interval, 1.03–1.32; P = 0.01), and lesionvolume showed a positive correlation with IgG level (r = 0.643, P < 0.001).
Conclusion
At 18-month follow-up assessments, 31.0% of participants exhibited residual CT abnormalities. Age and higher SARS-CoV-2 IgG levels were significant predictors, and FVC was related to abnormal CT findings at 18 months. Lesionvolume and FVC improved between 6 and 18 months.
Figure
Reference
-
1. Zhou F, Tao M, Shang L, Liu Y, Pan G, Jin Y, et al. Assessment of sequelae of COVID-19 nearly 1 year after diagnosis. Front Med (Lausanne). 2021; 8:717194. PMID: 34888318.2. Zhao Y, Yang C, An X, Xiong Y, Shang Y, He J, et al. Follow-up study on COVID-19 survivors one year after discharge from hospital. Int J Infect Dis. 2021; 112:173–182. PMID: 34520845.3. Bellan M, Baricich A, Patrucco F, Zeppegno P, Gramaglia C, Balbo PE, et al. Long-term sequelae are highly prevalent one year after hospitalization for severe COVID-19. Sci Rep. 2021; 11(1):22666. PMID: 34811387.4. Watanabe A, So M, Iwagami M, Fukunaga K, Takagi H, Kabata H, et al. One-year follow-up CT findings in COVID-19 patients: a systematic review and meta-analysis. Respirology. 2022; 27(8):605–616. PMID: 35694728.5. Faverio P, Luppi F, Rebora P, D’Andrea G, Stainer A, Busnelli S, et al. One-year pulmonary impairment after severe COVID-19: a prospective, multicenter follow-up study. Respir Res. 2022; 23(1):65. PMID: 35313890.6. Caruso D, Guido G, Zerunian M, Polidori T, Lucertini E, Pucciarelli F, et al. Post-acute sequelae of COVID-19 pneumonia: six-month chest CT follow-up. Radiology. 2021; 301(2):E396–E405. PMID: 34313468.7. Han X, Fan Y, Alwalid O, Li N, Jia X, Yuan M, et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology. 2021; 299(1):E177–E186. PMID: 33497317.8. Luger AK, Sonnweber T, Gruber L, Schwabl C, Cima K, Tymoszuk P, et al. Chest CT of lung injury 1 year after COVID-19 pneumonia: the CovILD study. Radiology. 2022; 304(2):462–470. PMID: 35348379.9. Bongiovanni M, Barilaro G, Bini F. Twelve-month clinical, functional, and radiological outcomes in patients hospitalized for SARS-CoV-2 pneumonia. J Med Virol. 2023; 95(2):e28524. PMID: 36695513.10. Lee JH, Yim JJ, Park J. Pulmonary function and chest computed tomography abnormalities 6-12 months after recovery from COVID-19: a systematic review and meta-analysis. Respir Res. 2022; 23(1):233. PMID: 36068582.11. Murphy MC, Little BP. Chronic pulmonary manifestations of COVID-19 infection: imaging evaluation. Radiology. 2023; 307(2):e222379. PMID: 36692398.12. Kanne JP, Little BP, Schulte JJ, Haramati A, Haramati LB. Long-term lung abnormalities associated with COVID-19 pneumonia. Radiology. 2023; 306(2):e221806. PMID: 36040336.13. Sul B, Flors L, Cassani J, Morris MJ, Reifman J, Altes T, et al. Volumetric characteristics of idiopathic pulmonary fibrosis lungs: computational analyses of high-resolution computed tomography images of lung lobes. Respir Res. 2019; 20(1):216. PMID: 31604436.14. Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, et al. Standardization of spirometry 2019 update. An Official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med. 2019; 200(8):e70–e88. PMID: 31613151.15. Guo Y, Wang H, Xiao M, Guan X, Lei Y, Diao T, et al. Long-term outcomes of COVID-19 convalescents: an 18.5-month longitudinal study in Wuhan. Int J Infect Dis. 2023; 127:85–92. PMID: 36509334.16. Barini M, Percivale I, Danna P, Longo V, Costantini P, Paladini A, et al. 18 Months computed tomography follow-up after Covid-19 interstitial pneumonia. J Public Health Res. 2022; 11(2):2782. PMID: 35315262.17. Solomon JJ, Heyman B, Ko JP, Condos R, Lynch DA. CT of post-acute lung complications of COVID-19. Radiology. 2021; 301(2):E383–E395. PMID: 34374591.18. Vijayakumar B, Tonkin J, Devaraj A, Philip KE, Orton CM, Desai SR, et al. CT lung abnormalities after COVID-19 at 3 months and 1 year after hospital discharge. Radiology. 2022; 303(2):444–454. PMID: 34609195.19. Desai SR, Wells AU, Rubens MB, Evans TW, Hansell DM. Acute respiratory distress syndrome: CT abnormalities at long-term follow-up. Radiology. 1999; 210(1):29–35. PMID: 9885583.20. Huntley CC, Patel K, Bil Bushra SE, Mobeen F, Armitage MN, Pye A, et al. Pulmonary function test and computed tomography features during follow-up after SARS, MERS and COVID-19: a systematic review and meta-analysis. ERJ Open Res. 2022; 8(2):00056-2022. PMID: 35642193.21. Chen Y, Ding C, Yu L, Guo W, Feng X, Yu L, et al. One-year follow-up of chest CT findings in patients after SARS-CoV-2 infection. BMC Med. 2021; 19(1):191. PMID: 34365975.22. Zhang P, Li J, Liu H, Han N, Ju J, Kou Y, et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res. 2020; 8(1):8. PMID: 32128276.23. Burnham EL, Hyzy RC, Paine R 3rd, Coley C 2nd, Kelly AM, Quint LE, et al. Chest CT features are associated with poorer quality of life in acute lung injury survivors. Crit Care Med. 2013; 41(2):445–456. PMID: 23263616.24. Xie L, Liu Y, Xiao Y, Tian Q, Fan B, Zhao H, et al. Follow-up study on pulmonary function and lung radiographic changes in rehabilitating severe acute respiratory syndrome patients after discharge. Chest. 2005; 127(6):2119–2124. PMID: 15947329.25. González J, Benítez ID, Carmona P, Santisteve S, Monge A, Moncusí-Moix A, et al. Pulmonary function and radiologic features in survivors of critical COVID-19: a 3-month prospective cohort. Chest. 2021; 160(1):187–198. PMID: 33676998.26. Wu X, Liu X, Zhou Y, Yu H, Li R, Zhan Q, et al. 3-Month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021; 9(7):747–754. PMID: 33964245.27. van der Sar-van der Brugge S, Talman S, Boonman-de Winter L, de Mol M, Hoefman E, van Etten RW, et al. Pulmonary function and health-related quality of life after COVID-19 pneumonia. Respir Med. 2021; 176:106272. PMID: 33302142.28. Nopp S, Moik F, Klok FA, Gattinger D, Petrovic M, Vonbank K, et al. Outpatient pulmonary rehabilitation in patients with long COVID improves exercise capacity, functional status, dyspnea, fatigue, and quality of life. Respiration. 2022; 101(6):593–601. PMID: 35203084.29. Carsetti R, Zaffina S, Piano Mortari E, Terreri S, Corrente F, Capponi C, et al. Different innate and adaptive immune responses to SARS-CoV-2 infection of asymptomatic, mild, and severe cases. Front Immunol. 2020; 11:610300. PMID: 33391280.30. Liu W, Fontanet A, Zhang PH, Zhan L, Xin ZT, Baril L, et al. Two-year prospective study of the humoral immune response of patients with severe acute respiratory syndrome. J Infect Dis. 2006; 193(6):792–795. PMID: 16479513.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characteristics and Prevalence of Sequelae after COVID-19: A Longitudinal Cohort Study
- Pulmonary Function, Functional Capacity, Respiratory, and Locomotor Muscle Strength after Severe to Critically Ill COVID-19: A Long-Term Study
- Evaluation and follow-up of pain, fatigue, and quality of life in COVID-19 patients
- A Case of Papulopustular Rosacea-Like Eruptions Following COVID-19 Vaccination
- The Impact of the Coronavirus Disease-2019 Pandemic on Childhood Obesity and Vitamin D Status