Clin Endosc.
2024 Jul;57(4):486-494. 10.5946/ce.2023.258.
Clinicopathological and endoscopic features of Helicobacter pylori infection-negative gastric cancer in Japan: a retrospective study
- Affiliations
-
- 1Department of Endoscopy, Fukuoka University Chikushi Hospital, Fukuoka, Japan
- 2Department of Pathology, Fukuoka University Chikushi Hospital, Fukuoka, Japan
- 3Department of Gastroenterology, Fukuoka University Chikushi Hospital, Fukuoka, Japan
- KMID: 2558105
- DOI: http://doi.org/10.5946/ce.2023.258
Abstract
- Background/Aims
Helicobacter pylori infection-negative gastric cancer (HPNGC) has not been systematically investigated in consecutive patients. Hence, this study aimed to investigate the clinicopathological and endoscopic features of HPNGC.
Methods
This single-center retrospective study selected participants from patients with gastric cancer who were treated at the Fukuoka University Chikushi Hospital between January 2013 and December 2021. Only patients diagnosed with HPNGC were enrolled, and their clinicopathological and endoscopic features were analyzed in detail.
Results
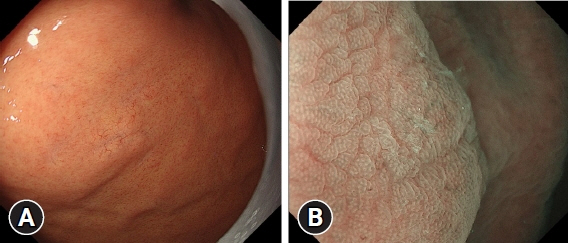
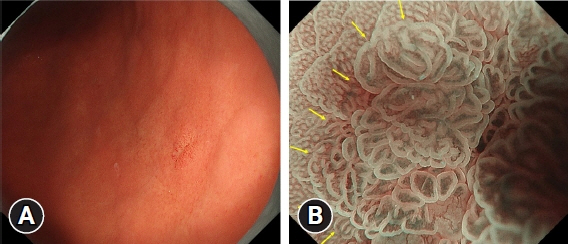
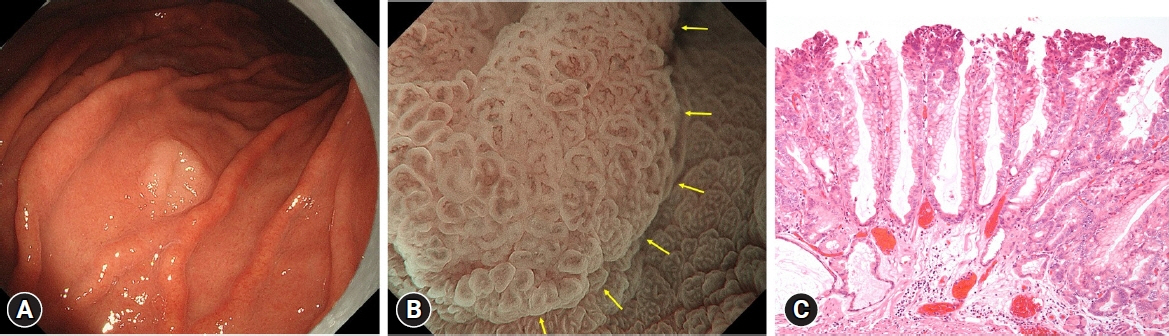
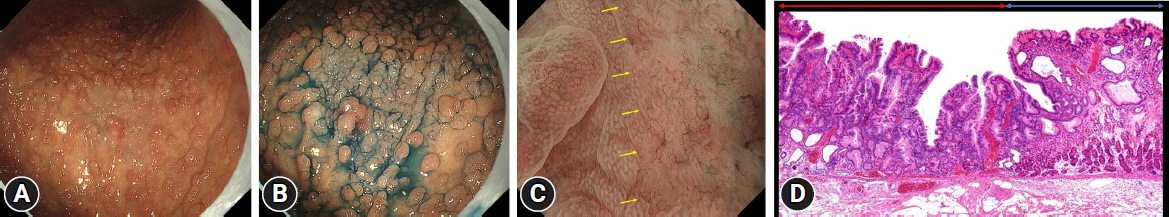
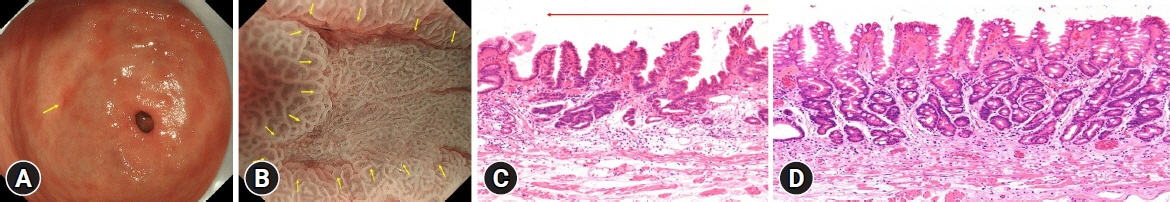
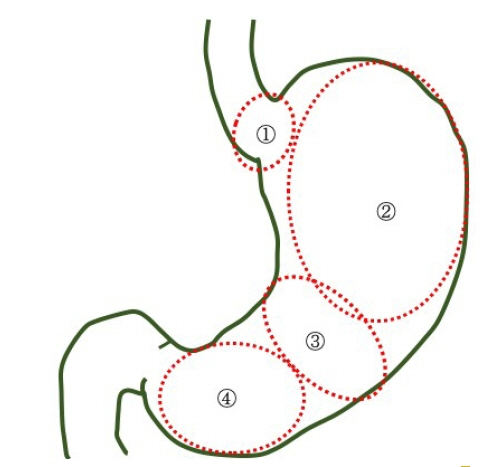
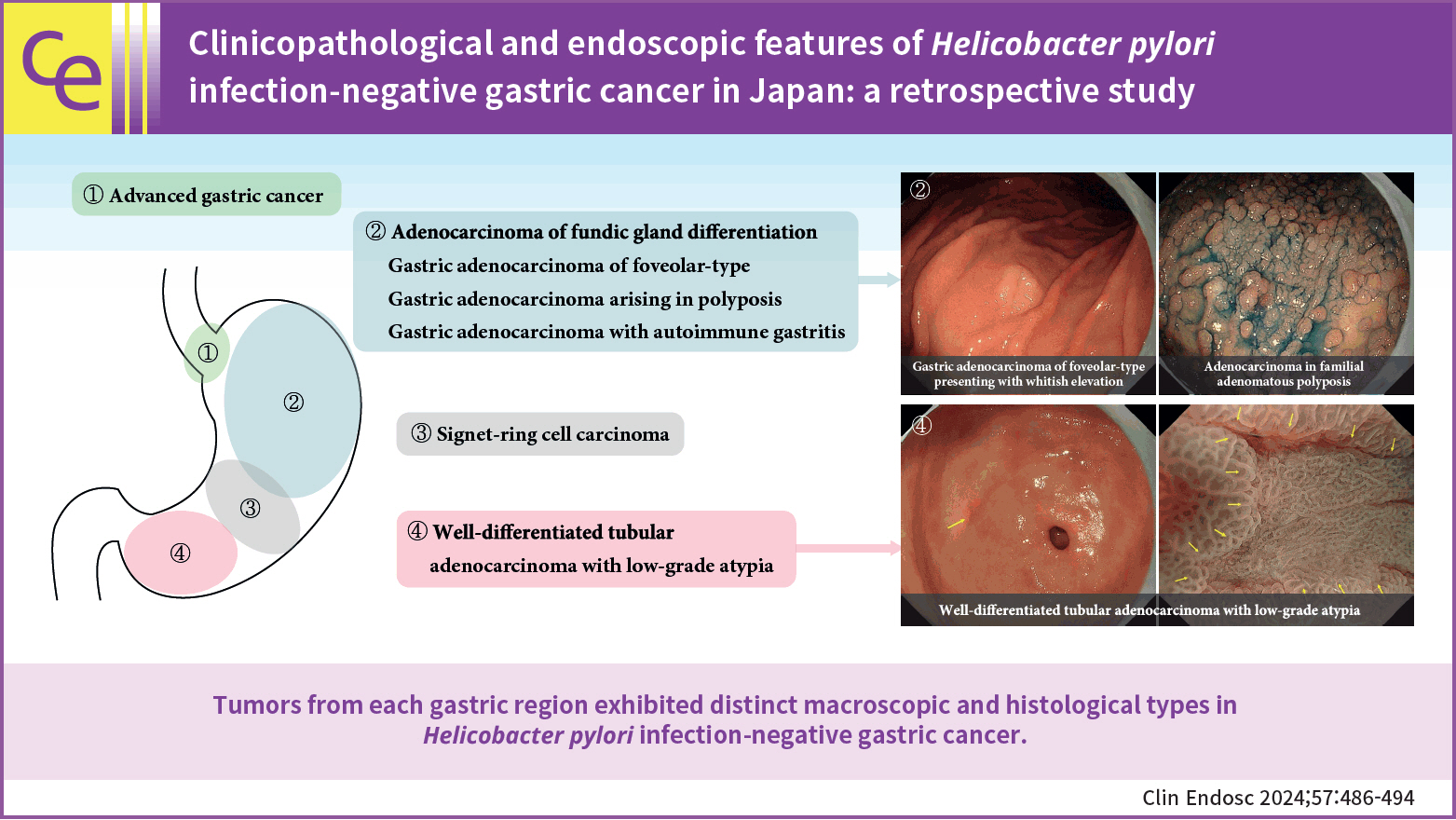
The prevalence of HPNGC in the present study was 2.6% (54/2112). The types of HPNGC observed in each gastric region were as follows: advanced gastric cancer was observed in the cardia; gastric adenocarcinoma of fundic-gland differentiation, gastric adenocarcinoma of foveolar-type presenting with whitish elevation and raspberry-like foveolar-type gastric adenocarcinoma, gastric adenocarcinoma arising in polyposis, and gastric adenocarcinoma with autoimmune gastritis were observed in the fundic gland region ranging from the gastric fornix to the gastric body; signet-ring cell carcinoma was observed in the gastric-pyloric transition region ranging from the lower gastric body to the gastric angle; and well-differentiated tubular adenocarcinoma with low-grade atypia was observed in the antrum.
Conclusions
This study revealed that tumors from each gastric region exhibited distinct macroscopic and histological types in HPNGC.
Figure
Cited by 1 articles
-
Is your endoscopist qualified enough to detect
Helicobacter pylori -naive status?
Sun-Young Lee
Clin Endosc. 2024;57(4):466-467. doi: 10.5946/ce.2024.033.
Reference
-
1. Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983; 1:1273–1275.2. Infection with Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. 1994; 61:177–240.3. Kamada T, Haruma K, Ito M, et al. Time trends in Helicobacter pylori infection and atrophic gastritis over 40 years in Japan. Helicobacter. 2015; 20:192–198.4. Ono S, Kato M, Suzuki M, et al. Frequency of Helicobacter pylori -negative gastric cancer and gastric mucosal atrophy in a Japanese endoscopic submucosal dissection series including histological, endoscopic and serological atrophy. Digestion. 2012; 86:59–65.
Article5. Kim HJ, Kim N, Yoon H, et al. Comparison between resectable Helicobacter pylori-negative and -positive gastric cancers. Gut Liver. 2016; 10:212–219.
Article6. Yamada A, Kaise M, Inoshita N, et al. Characterization of Helicobacter pylori-naïve early gastric cancers. Digestion. 2018; 98:127–134.
Article7. Sato C, Hirasawa K, Tateishi Y, et al. Clinicopathological features of early gastric cancers arising in Helicobacter pylori uninfected patients. World J Gastroenterol. 2020; 26:2618–2631.
Article8. Akazawa Y, Ueyama H, Hayashi T, et al. Clinicopathological and molecular characterization of early gastric adenocarcinoma in Helicobacter pylori-uninfected patients: emphasis on differentiated gastric adenocarcinoma. J Gastroenterol. 2022; 57:725–734.
Article9. Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969; 1:87–97.
Article10. Sakaki N, Momma K, Egawa N, et al. The influence of Helicobacter pylori infection on the progression of gastric mucosal atrophy and occurrence of gastric cancer. Eur J Gastroenterol Hepatol. 1995; 7 Suppl 1:S59–S62.11. Yao K. The endoscopic diagnosis of early gastric cancer. Ann Gastroenterol. 2013; 26:11–22.12. Yao K, Anagnostopoulos GK, Ragunath K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy. 2009; 41:462–467.
Article13. Yoon H, Kim N, Lee HS, et al. Helicobacter pylori-negative gastric cancer in South Korea: incidence and clinicopathologic characteristics. Helicobacter. 2011; 16:382–388.
Article14. Okano A, Kato S, Ohana M. Helicobacter pylori-negative gastric cancer: advanced-stage undifferentiated adenocarcinoma located in the pyloric gland area. Clin J Gastroenterol. 2017; 10:13–17.
Article15. Hayashi J, Yamatsuji T, Suehiro M, et al. Helicobacter pylori-negative advanced gastric cancer arising from the gastric mucosa without inflammation, atrophy, or intestinal metaplasia. Case Rep Gastroenterol. 2022; 16:345–350.
Article16. Takagi A, Ozawa H, Oki M, et al. Helicobacter pylori-negative advanced gastric cancer with massive eosinophilia. Intern Med. 2018; 57:1715–1718.
Article17. Ueyama H, Yao T, Nakashima Y, et al. Gastric adenocarcinoma of fundic gland type (chief cell predominant type): proposal for a new entity of gastric adenocarcinoma. Am J Surg Pathol. 2010; 34:609–619.
Article18. Ueyama H, Yao T, Akazawa Y, et al. Gastric epithelial neoplasm of fundic-gland mucosa lineage: proposal for a new classification in association with gastric adenocarcinoma of fundic-gland type. J Gastroenterol. 2021; 56:814–828.
Article19. Imamura K, Yao K, Nimura S, et al. Characteristic endoscopic findings of gastric adenocarcinoma of fundic-gland mucosa type. Gastric Cancer. 2021; 24:1307–1319.
Article20. Kanesaka T, Uedo N, Yao K, et al. New subtype of gastric adenocarcinoma: mixed fundic and pyloric mucosa-type adenocarcinoma. Clin J Gastroenterol. 2017; 10:224–228.
Article21. Takahashi H, Yao K, Ueo T, et al. Histological subtype of gastric adenocarcinoma: two cases of mixed fundic and pyloric mucosa-type adenocarcinoma. Ecancermedicalscience. 2020; 14:1143.
Article22. Shibagaki K, Fukuyama C, Mikami H, et al. Gastric foveolar-type adenomas endoscopically showing a raspberry-like appearance in the Helicobacter pylori-uninfected stomach. Endosc Int Open. 2019; 7:E784–E791.
Article23. Arai J, Niikura R, Hayakawa Y, et al. Clinicopathological features of gastric cancer with autoimmune gastritis. Biomedicines. 2022; 10:884.
Article24. Nikaido M, Kakiuchi N, Miyamoto S, et al. Indolent feature of Helicobacter pylori-uninfected intramucosal signet ring cell carcinomas with CDH1 mutations. Gastric Cancer. 2021; 24:1102–1114.
Article25. Muraishi J, Miyaoka M, Imamura K, et al. A case of gastric signet-ring cell carcinoma with a long-term retrospective follow-up of 17 years. Clin J Gastroenterol. 2021; 14:1337–1343.
Article26. Takita M, Ohata K, Inamoto R, et al. Endoscopic and histological features of Helicobacter pylori-negative differentiated gastric adenocarcinoma arising in the antrum. JGH Open. 2021; 5:470–477.
Article27. Kushima R, Nimura S. Stomach. In : Fukayama M, Morinaga S, editors. Surgical pathology. 5th ed. Bunkodo;2020. p. 444–515.28. Matsuhisa T, Tsukui T. Relation between reflux of bile acids into the stomach and gastric mucosal atrophy, intestinal metaplasia in biopsy specimens. J Clin Biochem Nutr. 2012; 50:217–221.
Article29. Tatsugami M, Ito M, Tanaka S, et al. Bile acid promotes intestinal metaplasia and gastric carcinogenesis. Cancer Epidemiol Biomarkers Prev. 2012; 21:2101–2107.
Article30. Matsuhisa T, Arakawa T, Watanabe T, et al. Relation between bile acid reflux into the stomach and the risk of atrophic gastritis and intestinal metaplasia: a multicenter study of 2283 cases. Dig Endosc. 2013; 25:519–525.
Article31. Li D, Zhang J, Yao WZ, et al. The relationship between gastric cancer, its precancerous lesions and bile reflux: a retrospective study. J Dig Dis. 2020; 21:222–229.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effectiveness of Helicobacter pylori Eradication before Endoscopic Resection
- Endoscopic Findings of Common Gastritis in Koreans
- Helicobacter pylori-negative Gastric Cancer
- Helicobacter pylori-negative Gastric Mucosa-associated Lymphoid Tissue Lymphoma
- Endoscopic Diagnosis of Early Gastric Cancer and High-Risk Gastritis