J Pathol Transl Med.
2024 Jul;58(4):165-173. 10.4132/jptm.2024.06.11.
Welcoming the new, revisiting the old: a brief glance at cytopathology reporting systems for lung, pancreas, and thyroid
- Affiliations
-
- 1Department of Pathology, Unidade Local de Saúde São José, Lisbon, Portugal
- 2Pathology Institute, Lisbon School of Medicine, Lisbon, Portugal
- 3Department of Pathology, All India Institute of Medical Sciences, New Delhi, India
- 4Instituto de Investigação e Inovação em Saúde (i3S), University of Porto, Porto, Portugal
- 5Institute of Molecular Pathology and Immunology of the University of Porto (Ipatimup), Porto, Portugal
- 6Abel Salazar Institute of Biomedical Sciences (ICBAS), University of Porto, Porto, Portugal
- KMID: 2557756
- DOI: http://doi.org/10.4132/jptm.2024.06.11
Abstract
- This review addresses new reporting systems for lung and pancreatobiliary cytopathology as well as the most recent edition of The Bethesda Reporting System for Thyroid Cytopathology. The review spans past, present, and future aspects within the context of the intricate interplay between traditional morphological assessments and cutting-edge molecular diagnostics. For lung and pancreas, the authors discuss the evolution of reporting systems, emphasizing the bridge between past directives and more recent collaborative efforts of the International Academy of Cytology and the World Health Organization in shaping universal reporting systems. The review offers a brief overview of the structure of these novel systems, highlighting their strengths and pinpointing areas that require further refinement. For thyroid, the authors primarily focus on the third edition of The Bethesda System for Reporting Thyroid Cytopathology, also considering the two preceding editions. This review serves as an invaluable resource for cytopathologists, offering a panoramic view of the evolving landscape of cytopathology reporting and pointing out the integrative role of the cytopathologist in an era of rapid diagnostic and therapeutic advancements.
Keyword
Figure
Reference
-
References
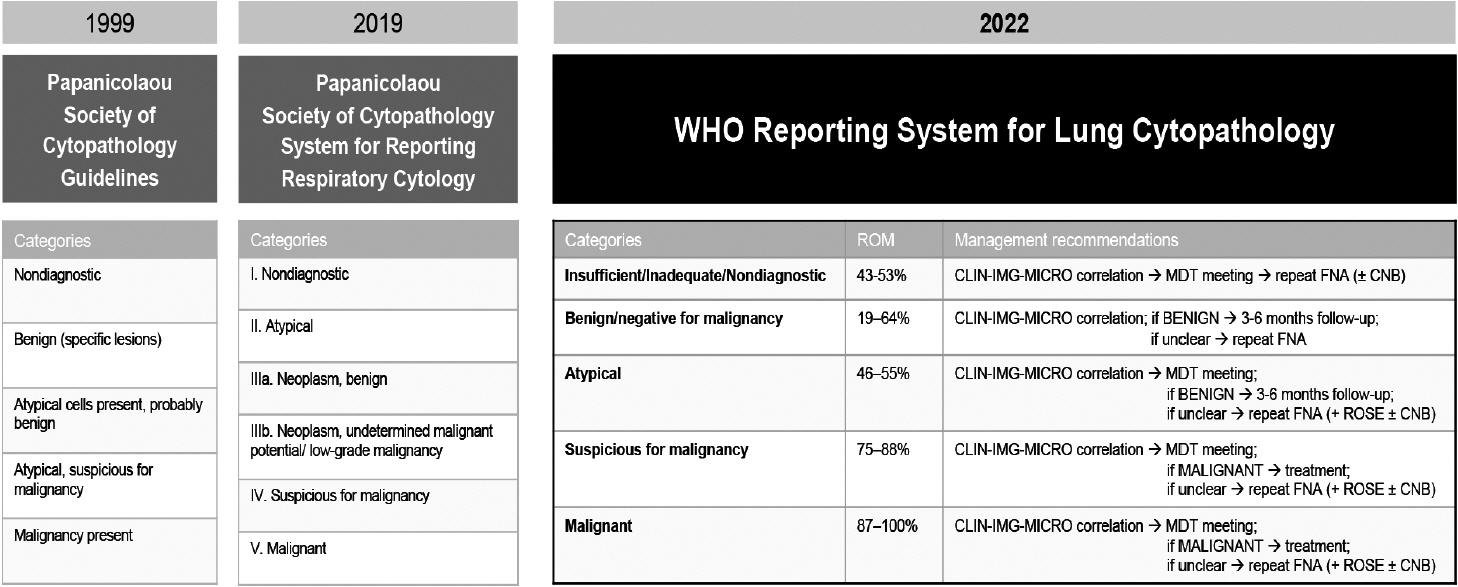
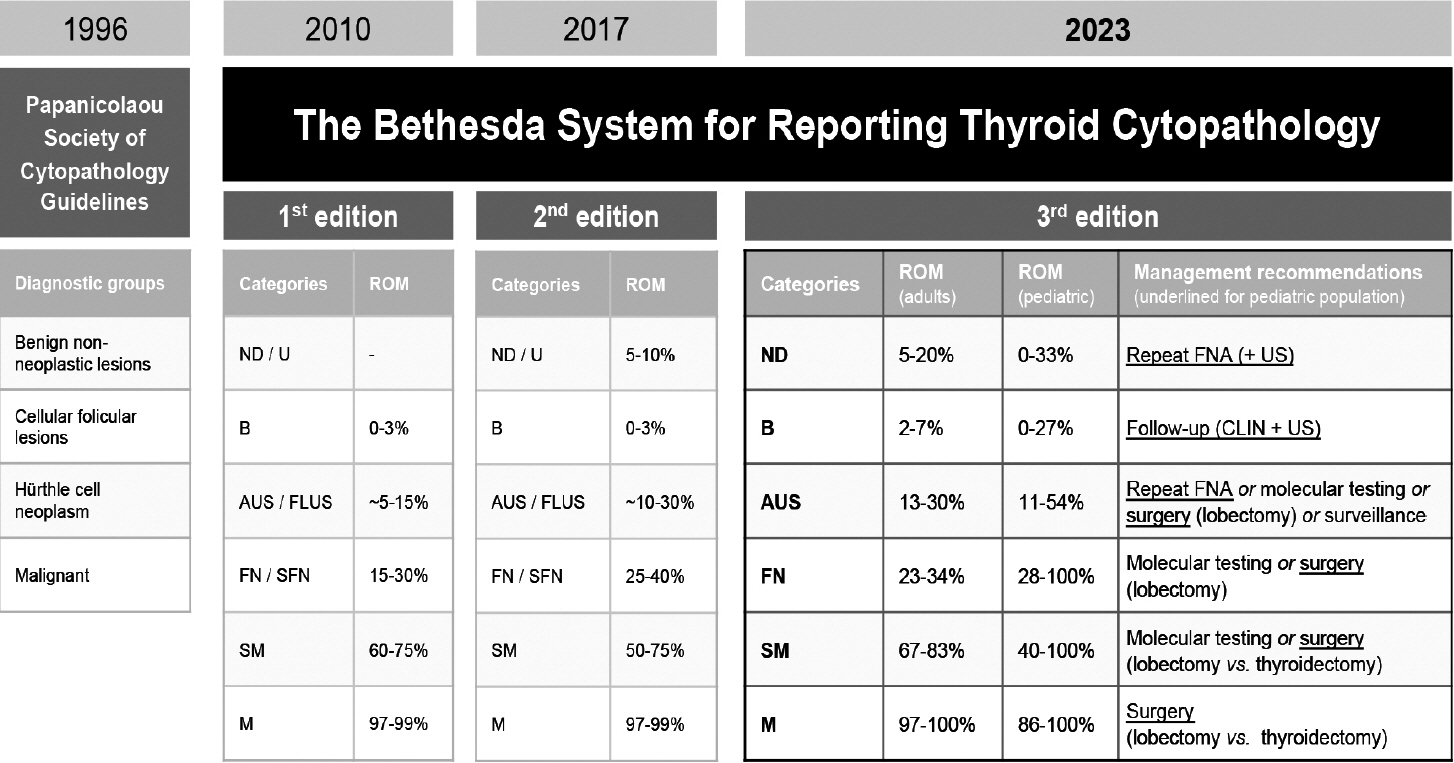
1. Layfield LJ, Baloch Z, Elsheikh T, et al. Standardized terminology and nomenclature for respiratory cytology: the Papanicolaou Society of Cytopathology guidelines. Diagn Cytopathol. 2016; 44:399–409.2. Hiroshima K, Yoshizawa A, Takenaka A, et al. Cytology reporting system for lung cancer from the Japan Lung Cancer Society and Japanese Society of Clinical Cytology: an interobserver reproducibility study and risk of malignancy evaluation on cytology specimens. Acta Cytol. 2020; 64:452–62.3. Alves PM, Ferreira F, Oliveira T, Alves D, Canberk S, Schmitt FC. A new cytology staining method: a fast approach for rapid on-site evaluation on thyroid fine-needle aspiration cytology. Acta Cytol. 2023; 67:289–94.4. Ammanagi AS, Dombale VD, Patil SS. On-site toluidine blue staining and screening improves efficiency of fine-needle aspiration cytology reporting. Acta Cytol. 2012; 56:347–51.5. International Academy of Cytology - International Agency for Research on Cancer - World Health Organization Joint Editorial Board. IAC-IARC-WHO Cytopathology Reporting Systems. Vol. 1. WHO Reporting System for Lung Cytopathology. Lyon: International Agency for Research on Cancer;2022.6. Layfield LJ, Baloch Z. The Papanicolaou Society of Cytopathology System for Reporting Respiratory Cytology: definitions, criteria, explanatory notes, and recommendations for ancillary testing. Cham: Springer International Publishing;2019.7. Guidelines of the Papanicolaou Society of Cytopathology for the examination of cytologic specimens obtained from the respiratory tract. Papanicolaou Society of Cytopathology Task Force on Standards of Practice. Diagn Cytopathol. 1999; 21:61–9.8. Canberk S, Montezuma D, Aydin O, et al. The new guidelines of Papanicolaou Society of Cytopathology for respiratory specimens: assessment of risk of malignancy and diagnostic yield in different cytological modalities. Diagn Cytopathol. 2018; 46:725–9.9. Hewer E, Schmitt AM. Ultrafast toluidine blue staining for rapid on-site evaluation of cytological smears. Acta Cytol. 2020; 64:375–7.10. WHO Classification of Tumours Editorial Board. Digestive system tumours. 5th ed. Vol. 1 [Internet]. Lyon: International Agency for Research on Cancer, 2019 [cited 2024 Apr 29]. Available from: https://publications.iarc.fr/579.11. Pitman MB, Centeno BA, Reid MD, et al. The World Health Organization reporting system for pancreaticobiliary cytopathology. Acta Cytol. 2023; 67:304–20.12. Pitman MB, Layfield LJ. Guidelines for pancreaticobiliary cytology from the Papanicolaou Society of Cytopathology: a review. Cancer Cytopathol. 2014; 122:399–411.13. Hoda RS, Arpin RN 3rd, Rosenbaum MW, Pitman MB. Risk of malignancy associated with diagnostic categories of the proposed World Health Organization International System for Reporting Pancreaticobiliary Cytopathology. Cancer Cytopathol. 2022; 130:195–201.14. Saieg M, Pitman MB. Experience and future perspectives on the use of the Papanicolaou Society of Cytopathology Terminology System for reporting pancreaticobiliary cytology. Diagn Cytopathol. 2020; 48:494–8.15. Ikemura K, Yan L, Park JW. Follow-up of indeterminate cytologic diagnoses of solid pancreatic lesions: atypia versus suspicious (one institution’s experience). J Am Soc Cytopathol. 2018; 7:160–5.16. Sung S, Del Portillo A, Gonda TA, Kluger MD, Tiscornia-Wasserman PG. Update on risk stratification in the Papanicolaou Society of Cytopathology System for Reporting Pancreaticobiliary Cytology categories: 3-year, prospective, single-institution experience. Cancer Cytopathol. 2020; 128:29–35.17. Pitman MB, Centeno BA, Reid MD, et al. A brief review of the WHO reporting system for pancreaticobiliary cytopathology. J Am Soc Cytopathol. 2023; 12:243–50.18. Pitman MB, Centeno BA, Genevay M, Fonseca R, Mino-Kenudson M. Grading epithelial atypia in endoscopic ultrasound-guided fineneedle aspiration of intraductal papillary mucinous neoplasms: an international interobserver concordance study. Cancer Cytopathol. 2013; 121:729–36.19. Cizginer S, Turner BG, Bilge AR, Karaca C, Pitman MB, Brugge WR. Cyst fluid carcinoembryonic antigen is an accurate diagnostic marker of pancreatic mucinous cysts. Pancreas. 2011; 40:1024–8.20. Singhi AD, Nikiforova MN, Fasanella KE, et al. Preoperative GNAS and KRAS testing in the diagnosis of pancreatic mucinous cysts. Clin Cancer Res. 2014; 20:4381–9.21. Lee JH, Kim Y, Choi JW, Kim YS. KRAS, GNAS, and RNF43 mutations in intraductal papillary mucinous neoplasm of the pancreas: a meta-analysis. Springerplus. 2016; 5:1172.22. Goyal A, Abdul-Karim FW, Yang B, Patel JB, Brainard JA. Interobserver agreement in the cytologic grading of atypia in neoplastic pancreatic mucinous cysts with the 2-tiered approach. Cancer Cytopathol. 2016; 124:909–16.23. Laquiere AE, Lagarde A, Napoleon B, et al. Genomic profile concordance between pancreatic cyst fluid and neoplastic tissue. World J Gastroenterol. 2019; 25:5530–42.24. Visani M, Acquaviva G, De Leo A, et al. Molecular alterations in pancreatic tumors. World J Gastroenterol. 2021; 27:2710–26.25. Goyal A, Sharaiha RZ, Alperstein SA, Siddiqui MT. Cytologic diagnosis of adenocarcinoma on bile duct brushings in the presence of stent associated changes: a retrospective analysis. Diagn Cytopathol. 2018; 46:826–32.26. Singhi AD, McGrath K, Brand RE, et al. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut. 2018; 67:2131–41.27. Younan G. Pancreas solid tumors. Surg Clin North Am. 2020; 100:565–80.28. Singhi AD, Nikiforova MN, Chennat J, et al. Integrating next-generation sequencing to endoscopic retrograde cholangiopancreatography (ERCP)-obtained biliary specimens improves the detection and management of patients with malignant bile duct strictures. Gut. 2020; 69:52–61.29. Ali SZ, Cibas ES. The Bethesda System for Reporting Thyroid Cytopathology: definitions, criteria and explanatory notes. New York: Springer;2010.30. Cibas ES, Ali SZ; NCI Thyroid FNA State of the Science Conference. The Bethesda System For Reporting Thyroid Cytopathology. Am J Clin Pathol. 2009; 132:658–65.31. The Papanicolaou Society of Cytopathology Task Force on Standards of Practice. Guidelines of the Papanicolaou Society of Cytopathology for the examination of fine-needle aspiration specimens from thyroid nodules. The Papanicolaou Society of Cytopathology Task Force on Standards of Practice. Diagn Cytopathol. 1998; 15:84–9.32. Ali SZ, Cibas ES. The Bethesda System for Reporting Thyroid Cytopathology: definitions, criteria and explanatory notes. Cham: Springer International Publishing;2018. 2nd.33. Ali SZ, VanderLaan PA. The Bethesda System for Reporting Thyroid Cytopathology: definitions, criteria and explanatory notes. New York: Springer International Publishing;2023. 3rd.34. Baloch Z, LiVolsi VA. The Bethesda System for Reporting Thyroid Cytology (TBSRTC): from look-backs to look-ahead. Diagn Cytopathol. 2020; 48:862–6.35. Ali SZ, Baloch ZW, Cochand-Priollet B, Schmitt FC, Vielh P, VanderLaan PA. The 2023 Bethesda system for reporting thyroid cytopathology. J Am Soc Cytopathol. 2023; 12:319–25.36. Juhlin CC, Mete O, Baloch ZW. The 2022 WHO classification of thyroid tumors: novel concepts in nomenclature and grading. Endocr Relat Cancer. 2023; 30:e220293.37. Kakudo K, Higuchi M, Hirokawa M, Satoh S, Jung CK, Bychkov A. Thyroid FNA cytology in Asian practice: active surveillance for indeterminate thyroid nodules reduces overtreatment of thyroid carcinomas. Cytopathology. 2017; 28:455–66.38. Kim M, Park HJ, Min HS, et al. The use of the Bethesda System for Reporting Thyroid Cytopathology in Korea: a nationwide multicenter survey by the Korean Society of Endocrine Pathologists. J Pathol Transl Med. 2017; 51:410–7.39. Vuong HG, Ngo HT, Bychkov A, et al. Differences in surgical resection rate and risk of malignancy in thyroid cytopathology practice between Western and Asian countries: a systematic review and meta-analysis. Cancer Cytopathol. 2020; 128:238–49.40. Ooi LY, Nga ME. Atypia of undetermined significance/follicular lesion of undetermined significance: Asian vs. non-Asian practice, and the Singapore experience. Gland Surg. 2020; 9:1764–87.41. Kakudo K, Jung CK, Liu Z, et al. The Asian Thyroid Working Group, from 2017 to 2023. J Pathol Transl Med. 2023; 57:289–304.42. Guerreiro SC, Tastekin E, Mourao M, et al. Impact of the 3rd edition of the Bethesda System for Reporting Thyroid Cytopathology on grey zone categories. Acta Cytol. 2023; 67:593–603.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Glance at the Bethesda System for Reporting Thyroid Cytopathology
- Thyroid Cytology in India: Contemporary Review and Meta-analysis
- Thyroid Imaging Reporting and Data System (TIRADS)
- What’s new in thyroid pathology 2024: updates from the new WHO classification and Bethesda system
- Highlights of the 2023 Bethesda System for Reporting Thyroid Cytopathology, 3rd Edition