J Pathol Transl Med.
2017 Nov;51(6):533-547. 10.4132/jptm.2017.08.04.
Thyroid Cytology in India: Contemporary Review and Meta-analysis
- Affiliations
-
- 1Department of Pathology, All India Institute of Medical Sciences, New Delhi, India. deepalijain76@gmail.com
- KMID: 2396535
- DOI: http://doi.org/10.4132/jptm.2017.08.04
Abstract
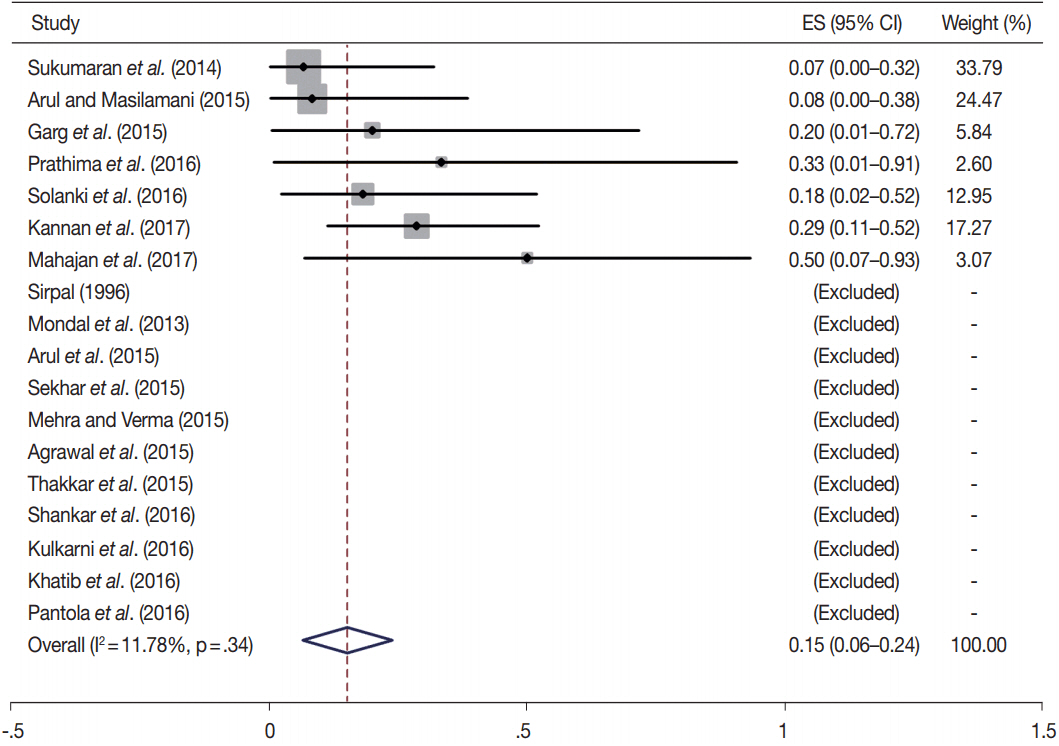
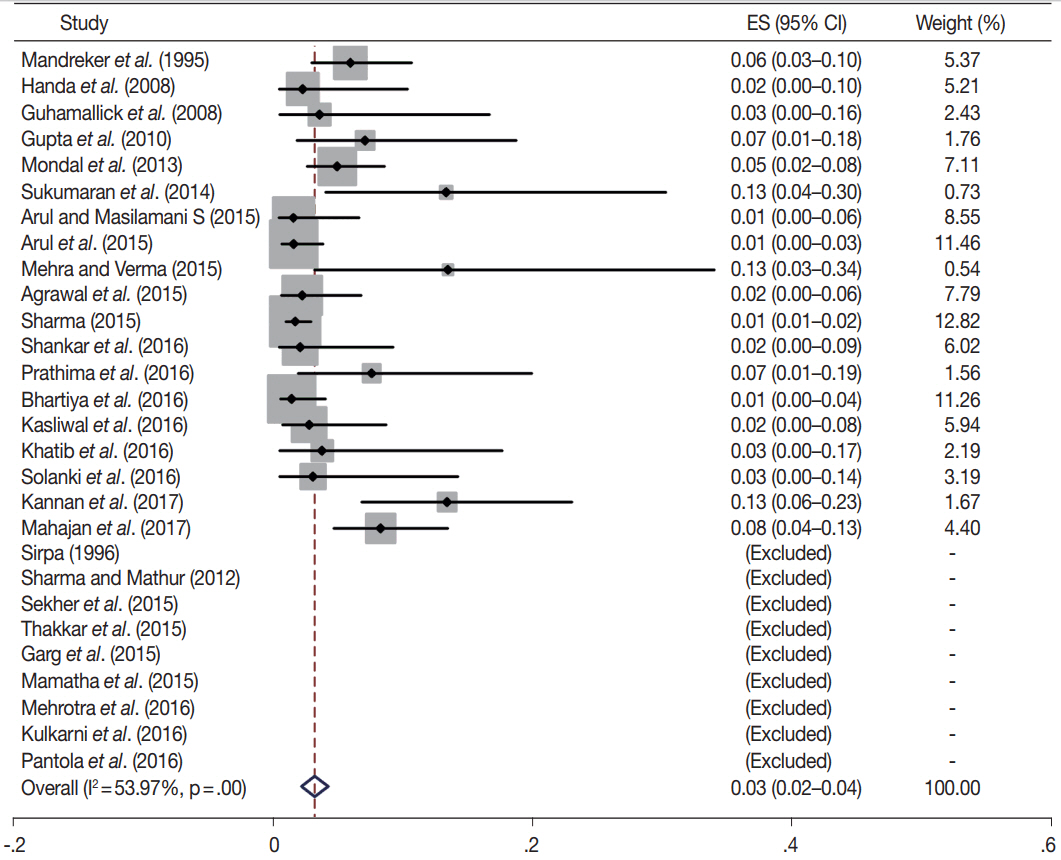
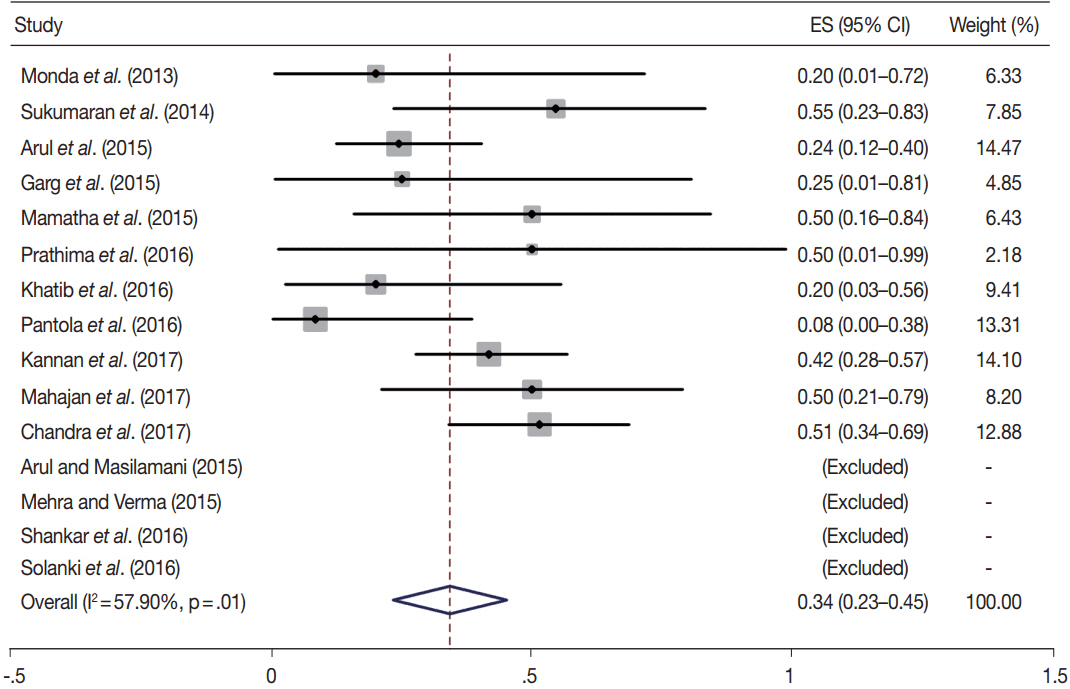
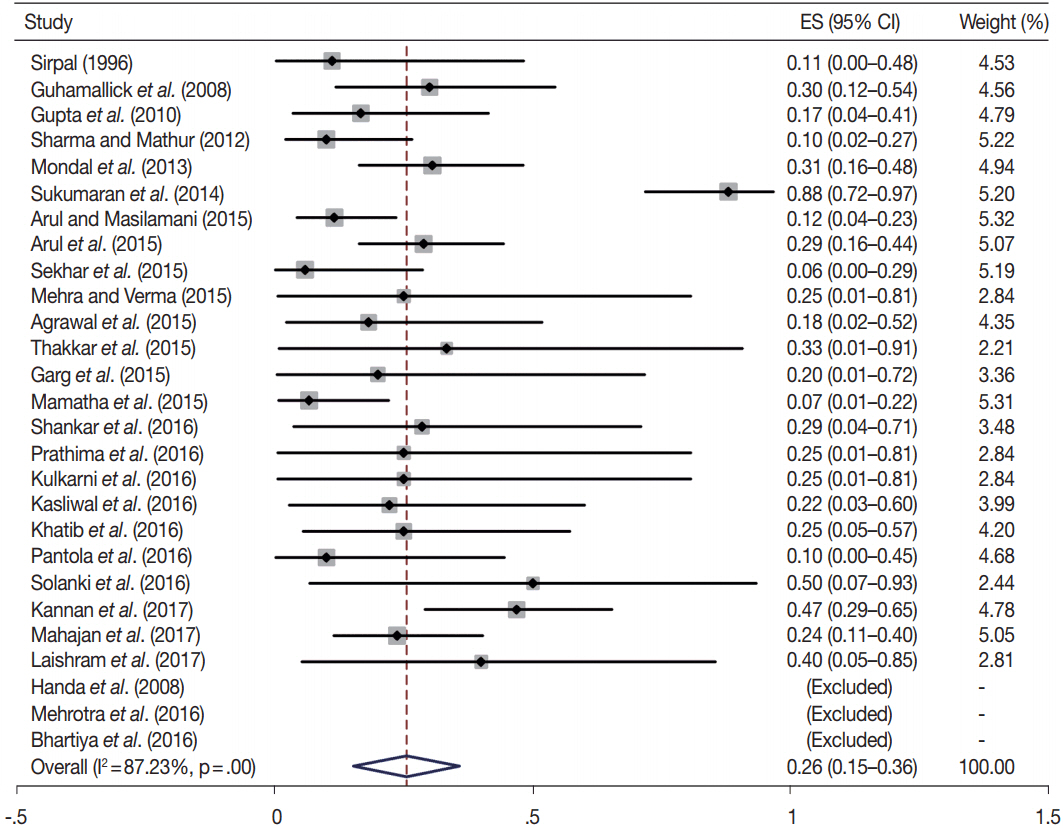
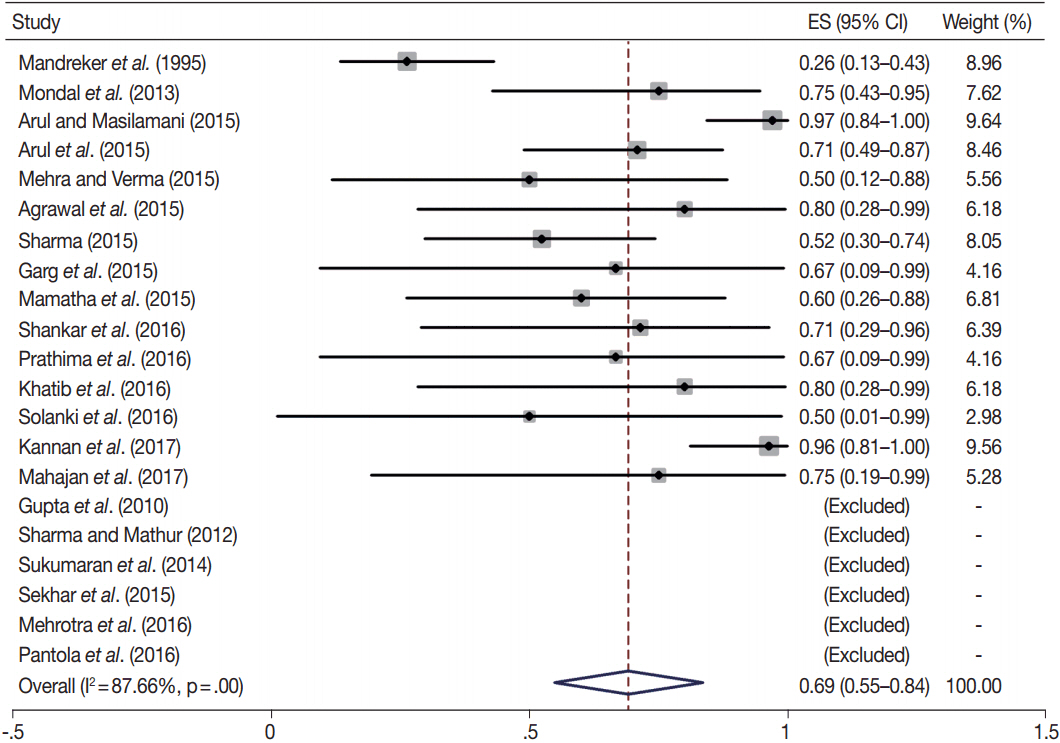
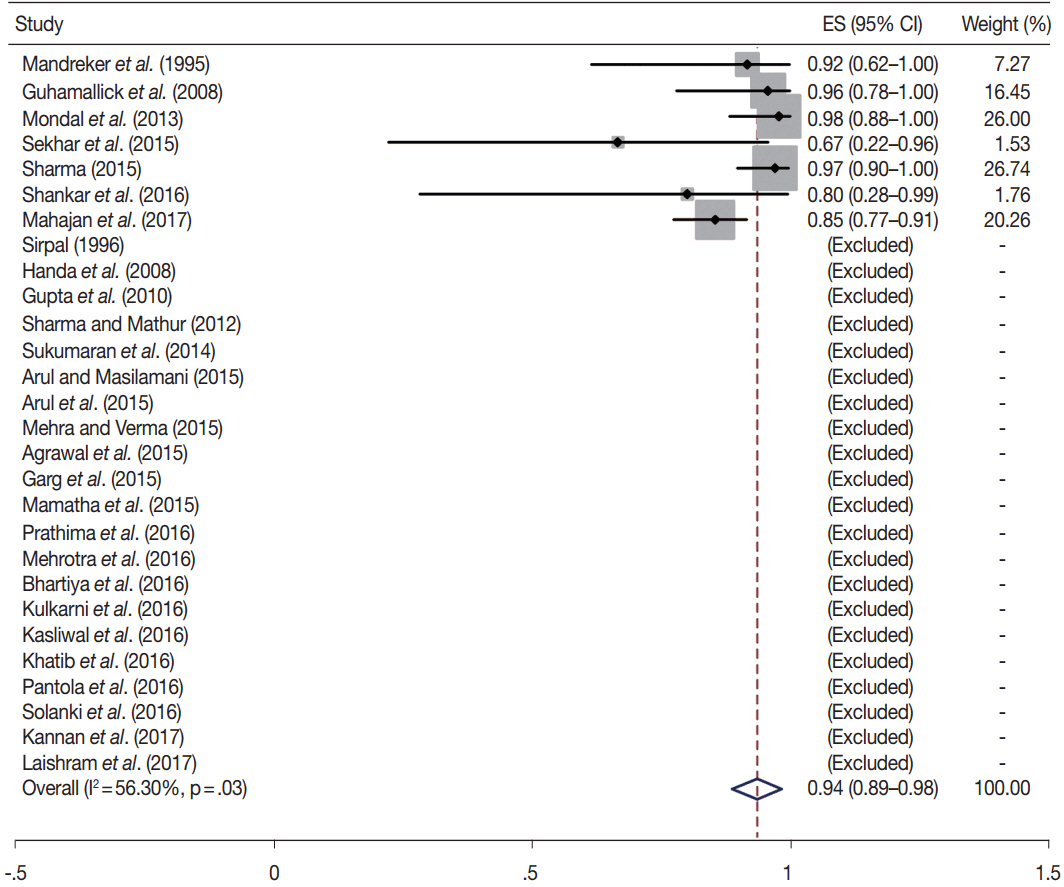
- Fine-needle aspiration cytology (FNAC) is a screening test for triaging thyroid nodules, aiding in subsequent clinical management. However, the advantages have been overshadowed by the multiplicity of reporting systems and a wide range of nomenclature used. The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) was formulated in 2007, to give the world a uniform thyroid cytology reporting system, facilitating easy interpretation by the clinicians. Here, we review the status of thyroid FNAC in India in terms of various reporting systems used including a meta-analysis of the previously published data. An extensive literature search was performed using internet search engines. The reports with detailed classification system used in thyroid cytology were included. The meta-analysis of published data was compared with the implied risk of malignancy by TBSRTC. More than 50 studies were retrieved and evaluated. TBSRTC is currently the most widely used reporting system with different studies showing good efficacy and interobserver concordance. Ancillary techniques have, as of now, limited applicability and acceptability in thyroid cytology in India. Twenty-eight published articles met the criteria for inclusion in the meta-analysis. When compared with TBSRTC recommendations, the meta-analysis showed a higher risk of malignancy for categories I and III. Thyroid FNAC is practiced all over India. TBSRTC has found widespread acceptance, with most institutions using this system for routine thyroid cytology reporting. However, reasons for a high malignancy risk for categories I and III need to be looked into. Various possible contributing factors are discussed in the review.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
The Use of Fine-Needle Aspiration (FNA) Cytology in Patients with Thyroid Nodules in Asia: A Brief Overview of Studies from the Working Group of Asian Thyroid FNA Cytology
Chan Kwon Jung, SoonWon Hong, Andrey Bychkov, Kennichi Kakudo
J Pathol Transl Med. 2017;51(6):571-578. doi: 10.4132/jptm.2017.10.19.The Asian Thyroid Working Group, from 2017 to 2023
Kennichi Kakudo, Chan Kwon Jung, Zhiyan Liu, Mitsuyoshi Hirokawa, Andrey Bychkov, Huy Gia Vuong, Somboon Keelawat, Radhika Srinivasan, Jen-Fan Hang, Chiung-Ru Lai
J Pathol Transl Med. 2023;57(6):289-304. doi: 10.4132/jptm.2023.10.04.
Reference
-
1. Gangadharan P, Nair MK, Pradeep VM. Thyroid cancer in Kerala. In : Shah AH, Samuel AM, Rao RS, editors. Thyroid cancer: an Indian perspective. Mumbai: Quest Publications;1999. p. 17–32.2. National Centre for Disease Informatics and Research. National Cancer Registry Programme Indian Council of Medical Research. Three-year report of population based cancer registries 2012-2014. Bengaluru: NCDIR-NCRP;2016.3. Unnikrishnan AG, Kalra S, Baruah M, et al. Endocrine Society of India management guidelines for patients with thyroid nodules: a position statement. Indian J Endocrinol Metab. 2011; 15:2–8.
Article4. Usha Menon V, Sundaram KR, Unnikrishnan AG, Jayakumar RV, Nair V, Kumar H. High prevalence of undetected thyroid disorders in an iodine sufficient adult south Indian population. J Indian Med Assoc. 2009; 107:72–7.5. Baloch ZW, LiVolsi VA, Asa SL, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008; 36:425–37.
Article6. Krauss EA, Mahon M, Fede JM, Zhang L. Application of the Bethesda classification for thyroid fine-needle aspiration: institutional experience and meta-analysis. Arch Pathol Lab Med. 2016; 140:1121–31.
Article7. Martin HE, Ellis EB. Biopsy by needle puncture and aspiration. Ann Surg. 1930; 92:169–81.
Article8. Soderstrom N. Puncture of goiters for aspiration biopsy. Acta Med Scand. 1952; 144:237–44.9. Das DK. Fine-needle aspiration cytology: its origin, development, and present status with special reference to a developing country, India. Diagn Cytopathol. 2003; 28:345–51.
Article10. Gupta SK, Dutta TK, Aikat M, Gupta BD, Talwar BL, Aikat BK. Evaluation of fine needle aspiration biopsy technique in the diagnosis of tumours. Indian J Cancer. 1975; 12:257–67.11. Singh P, Khanna SD, Manchanda RL. Needle biopsy of thyroid. Arch Surg. 1965; 91:646–51.
Article12. Rege JD, Nath AR, Bijlani JC, Trivedi DR, Deshpande DV. Fine needle aspiration cytology in solitary cold nodules of thyroid. J Assoc Physicians India. 1987; 35:819–21.13. Mandreker SR, Nadkarni NS, Pinto RG, Menezes S. Role of fine needle aspiration cytology as the initial modality in the investigation of thyroid lesions. Acta Cytol. 1995; 39:898–904.14. Sirpal YM. Efficacy of fine needle aspiration cytology in the management of thyroid diseases. Indian J Pathol Microbiol. 1996; 39:173–8.15. Handa U, Garg S, Mohan H, Nagarkar N. Role of fine needle aspiration cytology in diagnosis and management of thyroid lesions: a study on 434 patients. J Cytol. 2008; 25:13–7.
Article16. Guhamallick M, Sengupta S, Bhattacharya NK, et al. Cytodiagnosis of thyroid lesions: usefulness and pitfalls: a study of 288 cases. J Cytol. 2008; 25:6–9.17. Gupta M, Gupta S, Gupta VB. Correlation of fine needle aspiration cytology with histopathology in the diagnosis of solitary thyroid nodule. J Thyroid Res. 2010; 2010:379051.
Article18. Bagga PK, Mahajan NC. Fine needle aspiration cytology of thyroid swellings: how useful and accurate is it? Indian J Cancer. 2010; 47:437–42.
Article19. Sengupta A, Pal R, Kar S, Zaman FA, Sengupta S, Pal S. Fine needle aspiration cytology as the primary diagnostic tool in thyroid enlargement. J Nat Sci Biol Med. 2011; 2:113–8.20. Renuka IV, Saila Bala G, Aparna C, Kumari R, Sumalatha K. The Bethesda System for Reporting Thyroid Cytopathology: interpretation and guidelines in surgical treatment. Indian J Otolaryngol Head Neck Surg. 2012; 64:305–11.
Article21. Sharma R, Mathur DR. Diagnostic accuracy of fine needle aspiration cytology (FNAC) of the thyroid gland lesions. Int J Health Sci Res. 2012; 2:1–7.22. Patel MM, Patel K, Kaptan KR, Italiya SL, Saini G. Fine needle aspiration cytology as a first line investigation in thyroid lesions. Natl J Med Res. 2013; 3:106–10.23. Mondal SK, Sinha S, Basak B, Roy DN, Sinha SK. The Bethesda system for reporting thyroid fine needle aspirates: a cytologic study with histologic follow-up. J Cytol. 2013; 30:94–9.
Article24. Kukar N, Malhotra V, Saluja M. Analysis of fine needle aspiration cytology of thyroid lesions. Internet J Pathol. 2013; 15:1–9.25. Bhasin TS, Mannan R, Manjari M, et al. Reproducibility of ‘The Bethesda System for Reporting Thyroid Cytopathology’: a multicenter study with review of the literature. J Clin Diagn Res. 2013; 7:1051–4.
Article26. Borgohain R, Lal RK, Chatterjee P, Brahma N, Khanna S. A study of cyto-histological correlation in the diagnosis of thyroid swelling. IOSR J Dent Med Sci. 2014; 13:46–9.
Article27. Mangshetty SS, Jewargikar R, Andola SK. Fine needle aspiration cytology of 220 thyroid lesions with histopathological correlation. Int J Res Health Sci. 2014; 2:243–53.28. Panchal MG, Deshpande SA, Noone RB, Suvernakar SV, Meshram DP. Diagnostic accuracy fine needle aspiration cytology of thyroid gland lesions. Int J Pharm Sci Invent. 2014; 3:5–10.29. Pathak P, Srivastava R, Singh N, Arora VK, Bhatia A. Implementation of the Bethesda System for Reporting Thyroid Cytopathology: interobserver concordance and reclassification of previously inconclusive aspirates. Diagn Cytopathol. 2014; 42:944–9.
Article30. Sukumaran R, Kattoor J, Pillai KR, et al. Fine needle aspiration cytology of thyroid lesions and its correlation with histopathology in a series of 248 patients. Indian J Surg Oncol. 2014; 5:237–41.
Article31. Arul P, Masilamani S. A correlative study of solitary thyroid nodules using the Bethesda System for Reporting Thyroid Cytopathology. J Cancer Res Ther. 2015; 11:617–22.
Article32. Arul P, Akshatha C, Masilamani S. A study of malignancy rates in different diagnostic categories of the Bethesda System for Reporting Thyroid Cytopathology: an institutional experience. Biomed J. 2015; 38:517–22.
Article33. Sekhar A, Inamdae SS, Dombale VD, Prabhu MH. Fine needle aspiration cytology study of thyroid lesions: a 2 year prospective study in a tertiary centre. Int J Pharm Biol Sci Arch. 2015; 3:15–9.34. Mehra P, Verma AK. Thyroid cytopathology reporting by the Bethesda system: a two-year prospective study in an academic institution. Patholog Res Int. 2015; 2015:240505.
Article35. Agrawal R, Saxena M, Kumar P. A study of fine needle aspiration cytology of thyroid lesions with histopathological correlation. Indian J Pathol Oncol. 2015; 2:277–83.
Article36. Sharma C. Diagnostic accuracy of fine needle aspiration cytology of thyroid and evaluation of discordant cases. J Egypt Natl Canc Inst. 2015; 27:147–53.
Article37. Thakkar B, Patel PJ, Mangar U, Shiladariya P, Suthar NB, Patel P. Retrospective study of fine needle aspiration cytology of thyroid lesions according to the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC). Int J Res Med. 2015; 4:132–6.38. Garg S, Desai NJ, Mehta D, Vaishnav M. To establish Bethesda system for diagnosis of thyroid nodules on the basis of FNAC with histopathological correlation. J Clin Diagn Res. 2015; 9:EC17–21.39. Kathirvel C. Criteria for cytological diagnosis of thyroid lesions. Otolaryngol Online J. 2015; 5(Suppl 2):10–9.40. Alagarsamy J, Sivaraman R, Kadhirvel V. Accuracy of fine needle aspiration cytology and incidence of benign/malignant tumours in solitary thyroid nodules. Int J Allied Med Sci Clin Res. 2015; 3:193–9.41. Hathila R, Patel S, Vaghela P, Makwana G, Parmar P. Cytology findings of the thyroid lesions with the histopathology findings correlation. Int J Med Sci Public Health. 2016; 5:642–6.
Article42. Mamatha M, Sekhar SC, Rani HS, Anil SS, Vandana G. A comparative study between conventional system and the Bethesda system applied for reporting thyroid cytopathology. Int Arch Integr Med. 2015; 2:87–95.43. Gupta V, Bhake A, Dayal S. Pattern and frequency of thyroid pathologies among thyroid cytology specimen in rural part of central India: a retrospective secondary data analysis. Thyroid Res Pract. 2015; 12:93–5.
Article44. Shankar SP, Meenakshisundaram K, Rajalakshmi V, Selvakumar SA, Giridharan B. The Bethesda System for Reporting Thyroid Cytopathology: a two year retrospective review in a tertiary care hospital. Indian J Pathol Oncol. 2016; 3:48–54.
Article45. Prathima S, Suresh TN, Harendra Kumar ML, Bhaskaran A. Impact of the Bethesda system in reporting thyroid cytopathology. Thyroid Res Pract. 2016; 13:9–14.
Article46. Mehrotra D, Anita AM, Andola SK, Patil AG. Thyroid cytology evaluation based on the Bethesda system with clinico-morphological correlation. Ann Pathol Lab Med. 2016; 3:A347–355.47. Tagore S, Jayaprakash HT, Sasidharan A, Nagaraj T, Santosh HN, Balraj L. Cytological study of thyroid lesions by fine-needle aspiration cytology. J Med Radiol Pathol Surg. 2016; 2:12–5.
Article48. Kalita DJ, Das B. A three years study of FNAC of thyroid lesions in a tertiary care hospital. Indian J Appl Res. 2016; 6:559–61.49. Bhartiya R, Mallik M, Kumari N, Prasad BN. Evaluation of thyroid lesions by fine-needle aspiration cytology based on Bethesda System for Reporting Thyroid Cytopathology classification among the population of South Bihar. Indian J Med Paediatr Oncol. 2016; 37:265–70.
Article50. Kulkarni CV, Mittal M, Nema M, Verma R. Diagnostic role of the Bethesda System for Reporting Thyroid Cytopathology in an academic institute of Central India: one year experience. Indian J Basic Appl Med Res. 2016; 5:157–66.51. Lohiya V, Kalla AR, Bharadwaj V. Utilisation of Bethesda System for Reporting Thyroid Cytopathology: a study of 250 cases. Int J Appl Res. 2016; 2:103–7.52. Kasliwal N, Tanwar S, Pachori G, Gupta N, Maheshwari N, Jain D. Usefulness of preoperative FNAC of thyroid swelling along with application of Bethesda system of reporting. Int J Med Res Prof. 2016; 2:204–9.
Article53. Khatib Y, Mulla A, Patel RD, Momin E, Gite V, Khade A. Classification of thyroid FNA smears into Bethesda categories and their correlation with thyroid function tests. Sch J Appl Med Sci. 2016; 4:916–23.54. Pantola C, Kala S, Khan L, Pantola S, Singh M, Verma S. Cytological diagnosis of pediatric thyroid nodule in perspective of the Bethesda System for Reporting Thyroid Cytopathology. J Cytol. 2016; 33:220–3.
Article55. Babu SB, Raju R, Radhakrishnan S. Correlation of fine needle aspiration cytology with histopathology in the diagnosis of thyroid swellings. Int Surg J. 2016; 3:1437–41.
Article56. Solanki R, Chaudhary VK, Sharma M, Ansari M. Validity assessment of ‘the Bethesda System for Reporting Thyroid Cytopathology’. Int J Health Sci Res. 2016; 6:126–32.57. Aramani SS, Gururajaprasad C. A cytohistopathological correlation of thyroid lesions with critical evaluation of discordant cases: an experience at a tertiary care hospital. Ann Pathol Lab Med. 2017; 4:A243–8.
Article58. Sunder KS, Khan MI. Role of fine needle aspiration cytology (FNAC) in diagnosis of thyroid lesions. J Contemp Med Dent. 2017; 5:30–4.59. Garg S, Naik LP, Kothari KS, Fernandes GC, Agnihotri MA, Gokhale JC. Evaluation of thyroid nodules classified as Bethesda category III on FNAC. J Cytol. 2017; 34:5–9.
Article60. Kannan S, Raju N, Kekatpure V, et al. Improving Bethesda reporting in thyroid cytology: a team effort goes a long way and still miles to go. Indian J Endocrinol Metab. 2017; 21:277–81.
Article61. Mahajan S, Srinivasan R, Rajwanshi A, et al. Risk of malignancy and risk of neoplasia in the Bethesda indeterminate categories: study on 4,532 thyroid fine-needle aspirations from a single institution in India. Acta Cytol. 2017; 61:103–10.
Article62. Chandra S, Chandra H, Bisht SS. Malignancy rate in thyroid nodules categorized as atypia of undetermined significance or follicular lesion of undetermined significance: an institutional experience. J Cytol. 2017; 34:144–8.63. Laishram RS, Zothanmawii T, Joute Z, Yasung P, Debnath K. The Bethesda system of reporting thyroid fine needle aspirates: a 2-year cytologic study in a tertiary care institute. J Med Soc. 2017; 31:3–7.
Article64. Witt BL, Schmidt RL. Rapid onsite evaluation improves the adequacy of fine-needle aspiration for thyroid lesions: a systematic review and meta-analysis. Thyroid. 2013; 23:428–35.
Article65. Shield PW, Cosier J, Ellerby G, Gartrell M, Papadimos D. Rapid on-site evaluation of fine needle aspiration specimens by cytology scientists: a review of 3032 specimens. Cytopathology. 2014; 25:322–9.
Article66. Pitman MB, Abele J, Ali SZ, et al. Techniques for thyroid FNA: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008; 36:407–24.
Article67. Keyhani E, Sharghi SA, Amini R, et al. Liquid base cytology in evaluation of thyroid nodules. J Diabetes Metab Disord. 2014; 13:82.
Article68. Prasad D, Bhaskar V, Kumar B. Study of utility of manual liquid-based cytology and conventional smears in the evaluation of various fine-needle aspiration samples. Paripex Indian J Res. 2017; 6:609–10.69. Tripathy K, Misra A, Ghosh JK. Efficacy of liquid-based cytology versus conventional smears in FNA samples. J Cytol. 2015; 32:17–20.
Article70. Gupta V, Bhake A, Dayal S. Better thyroid cytopathology reporting and interpretation using different classification systems. Thyroid Res Pract. 2016; 13:110–4.
Article71. Bose S, Kapila K, Verma K. Medullary carcinoma of the thyroid: a cytological, immunocytochemical, and ultrastructural study. Diagn Cytopathol. 1992; 8:28–32.
Article72. Gupta A, Jain S, Khurana N, Kakar AK. Expression of p63 and Bcl-2 in malignant thyroid tumors and their correlation with other diagnostic immunocytochemical markers. J Clin Diagn Res. 2016; 10:EC04–8.
Article73. Tandon A, Paul TR, Singh R, Narendra AM. Synchronous thyroid involvement in plasma cell leukemia masquerading as Hashimoto’s thyroiditis: role of ancillary cytology techniques in diagnostic workup. Endocr Pathol. 2015; 26:324–7.
Article74. Goel MM, Budhwar P. Fine needle aspiration cytology and immunocytochemistry in tuberculous thyroiditis: a case report. Acta Cytol. 2008; 52:602–6.75. Aron M, Kapila K, Verma K. Utility of galectin 3 expression in thyroid aspirates as a diagnostic marker in differentiating benign from malignant thyroid neoplasms. Indian J Pathol Microbiol. 2006; 49:376–80.76. Jain D, Mathur SR, Guleria R, Iyer VK. Utility and pattern of positivity of p40 in the diagnosis of squamous cell carcinoma of the lung by cytology: the first study on fine needle aspiration smears. Cytopathology. 2014; 25:330–5.
Article77. Jain D, Mathur SR, Sharma MC, Iyer VK. Cytomorphology of sebaceous carcinoma with analysis of p40 antibody expression. Diagn Cytopathol. 2015; 43:456–61.
Article78. Vallonthaiel AG, Jain D, Singh V, et al. c-Myb overexpression in cytology smears of tracheobronchial and pulmonary adenoid cystic carcinomas. Acta Cytol. 2017; 61:77–83.
Article79. Roy M, Jain D, Bakhshi S, Mathur S, Iyer VK. Primary Langerhans cell histiocytosis of the thyroid gland: role of langerin in FNA cytological diagnosis. Cytopathology. 2015; 26:128–30.
Article80. Jain D, Mathur SR, Iyer VK. Cell blocks in cytopathology: a review of preparative methods, utility in diagnosis and role in ancillary studies. Cytopathology. 2014; 25:356–71.
Article81. Bhandari V, Yadav YK, Khanna G, Sharma M, Singh M. Efficacy of cytokeratin 19 expression on fine needle aspiration cell blocks in pre-operative diagnosis of malignant thyroid neoplasms. Clin Cancer Invest J. 2012; 1:212–6.82. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016; 26:1–133.83. Reddy KH, Kumar DR, Punnoose AM, et al. Detection of BRAF mutations in different thyroid cancers of archival FNAC samples. Indian J Endocrinol Metab. 2013; 17(Suppl 1):S373–394.84. Bychkov A. Prevalence of BRAFV600E mutation in Asian patients with thyroid cancer. Malays J Pathol. 2017; 39:95–6.85. Katna R, Shet T, Sengar M, et al. Clinicopathologic study and outcome analysis of thyroid lymphomas: experience from a tertiary cancer center. Head Neck. 2013; 35:165–71.
Article86. Usha M, Kamath S, Sridhar M, Soman S. Primary thyroid lymphoma in the background of Hashimoto thyroiditis. Clin Cancer Invest J. 2015; 4:362–4.87. Yeshvanth SK, Lakshminarayana KP, Upadhyaya VS, Shetty JK. Primary thyroid lymphoma arising from Hashimotos thyroiditis diagnosed by fine needle aspiration cytology. J Cancer Res Ther. 2012; 8:159–61.
Article88. Somani S, Kotwal N. Core needle biopsy: an additional diagnostic armamentarium. Thyroid Res Pract. 2016; 13:52–6.
Article89. Straccia P, Rossi ED, Bizzarro T, et al. A meta-analytic review of the Bethesda System for Reporting Thyroid Cytopathology: has the rate of malignancy in indeterminate lesions been underestimated? Cancer Cytopathol. 2015; 123:713–22.
Article90. Olson MT, Clark DP, Erozan YS, Ali SZ. Spectrum of risk of malignancy in subcategories of ‘atypia of undetermined significance’. Acta Cytol. 2011; 55:518–25.
Article91. Horne MJ, Chhieng DC, Theoharis C, et al. Thyroid follicular lesion of undetermined significance: evaluation of the risk of malignancy using the two-tier subclassification. Diagn Cytopathol. 2012; 40:410–5.
Article92. Kim SD, Han SH, Jeong WJ, Kim H, Ahn SH. Differences in clinical features between subcategories of “atypia/follicular lesion of undetermined significance”. Endocr Pathol. 2017; May. 9. [Epub]. https://doi.org/10.1007/s12022-017-9486-3.
Article93. Rosario PW, Calsolari MR. Importance of cytological subclassification of thyroid nodules with Bethesda category III cytology (AUS/FLUS) into architectural atypia only and nuclear atypia: a prospective study. Diagn Cytopathol. 2017; 45:604–7.
Article94. Layfield LJ, Baloch ZW, Esebua M, Kannuswamy R, Schmidt RL. Impact of the reclassification of the non-invasive follicular variant of papillary carcinoma as benign on the malignancy risk of the Bethesda System for Reporting Thyroid Cytopathology: a meta-analysis study. Acta Cytol. 2017; 61:187–93.
Article95. Bychkov A, Hirokawa M, Jung CK, et al. Low rate of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice. Thyroid. 2017; 27:983–4.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Use of Fine-Needle Aspiration (FNA) Cytology in Patients with Thyroid Nodules in Asia: A Brief Overview of Studies from the Working Group of Asian Thyroid FNA Cytology
- Significance of Lymphovascular Invasion as a Prognostic Factor in Patients with Papillary Thyroid Cancer: a Systematic Review and Meta-Analysis
- No Change in Complications Following Thyroidectomy Despite Increase in Thyroid Cancer Surgeries: A Meta-Regression Analysis
- Welcoming the new, revisiting the old: a brief glance at cytopathology reporting systems for lung, pancreas, and thyroid
- Diagnostic performance of ultrasound risk stratification systems on thyroid nodules cytologically classified as indeterminate: a systematic review and meta-analysis