Ann Rehabil Med.
2024 Jun;48(3):192-202. 10.5535/arm.240034.
Effects of Botulinum Toxin-A for Spasticity and Nociceptive Pain in Individuals with Spinal Cord Injury: A Systematic Review and Meta-Analysis
- Affiliations
-
- 1Department of Rehabilitation Science, Graduate School of Inje University, Gimhae, Korea
- 2Biohealth Products Research Center (BPRC), Inje University, Gimhae, Korea
- 3Research Center for Aged-life Redesign (RCAR), Inje University, Gimhae, Korea
- 4Department of Physical Therapy, College of Healthcare Medical Science & Engineering, Gimhae, Korea
- 5Department of Digital Anti-aging Healthcare, Graduate School of Inje University, Gimhae, Korea
- 6Department of Physical Therapy, Graduate School of Inje University, Gimhae, Korea
- 7Department of Rehabilitation Medicine, Medical Corporation, Daegu Medical Foundation The K Hospital, Daegu, Korea
- KMID: 2557066
- DOI: http://doi.org/10.5535/arm.240034
Abstract
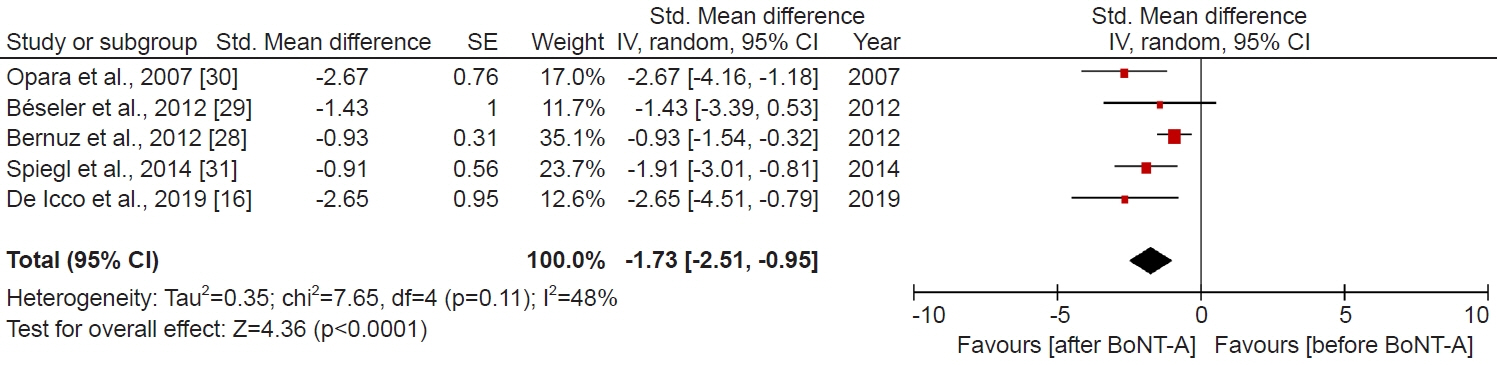
- We conducted a systematic review and meta-analysis to examine the protective effects of botulinum toxin-A (Botox-A) on spasticity and nociceptive pain in individuals with spinal cord injuries (SCIs). PubMed, Embase, and Cochrane Library databases were searched from inception to July 2023. The primary outcome of interest was spasticity and nociceptive pain. We pooled the available data using the generic inverse variance method, and we used a fixed-effect/random-effects model. We then calculated standardized mean difference (SMD) and 95% confidence intervals (95% CIs) to estimate the effect size. A total of fourteen studies meeting the inclusion criteria comprised two randomized controlled trials, five pre-post studies, and seven case reports. Across the various study designs, the majority of trials were assessed to have fair to high quality. The meta-analysis shows that Botox-A significantly decreased spasticity (SMD, -1.73; 95% CI, -2.51 to -0.95; p<0.0001, I2=48%) and nociceptive pain (SMD, -1.79; 95% CI, -2.67 to -0.91; p<0.0001, I2=0%) in SCI patients. Furthermore, Botox-A intervention improved motor function, activities of daily living (ADL), and quality of life. Our study suggests that Botox-A may alleviate spasticity and nociceptive pain in SCI patients. Moreover, the observed improvements in motor function, ADL, and overall quality of life following Botox-A intervention underscore its pivotal role in enhancing patient outcomes.
Keyword
Figure
Reference
-
1. Tibbett JA, Field-Fote EC, Thomas CK, Widerström-Noga EG. Spasticity and pain after spinal cord injury: impact on daily life and the influence of psychological factors. PM R. 2020; 12:119–29.
Article2. McKay WB, Sweatman WM, Field-Fote EC. The experience of spasticity after spinal cord injury: perceived characteristics and impact on daily life. Spinal Cord. 2018; 56:478–86.
Article3. Bryce TN, Biering-Sørensen F, Finnerup NB, Cardenas DD, Defrin R, Lundeberg T, et al. International spinal cord injury pain classification: part I. Background and description. March 6-7, 2009. Spinal Cord. 2012; 50:413–7.
Article4. Hunt C, Moman R, Peterson A, Wilson R, Covington S, Mustafa R, et al. Prevalence of chronic pain after spinal cord injury: a systematic review and meta-analysis. Reg Anesth Pain Med. 2021; 46:328–36.
Article5. Elbasiouny SM, Moroz D, Bakr MM, Mushahwar VK. Management of spasticity after spinal cord injury: current techniques and future directions. Neurorehabil Neural Repair. 2010; 24:23–33.
Article6. de Sousa N, Santos D, Monteiro S, Silva N, Barreiro-Iglesias A, Salgado AJ. Role of baclofen in modulating spasticity and neuroprotection in spinal cord injury. J Neurotrauma. 2022; 39:249–58.
Article7. Ward AB. Spasticity treatment with botulinum toxins. J Neural Transm (Vienna). 2008; 115:607–16.
Article8. Harvey LA, Glinsky JV, Bowden JL. The effectiveness of 22 commonly administered physiotherapy interventions for people with spinal cord injury: a systematic review. Spinal Cord. 2016; 54:914–23.
Article9. Palazón-García R, Alcobendas-Maestro M, Esclarin-de Ruz A, Benavente-Valdepeñas AM. Treatment of spasticity in spinal cord injury with botulinum toxin. J Spinal Cord Med. 2019; 42:281–7.
Article10. Palazón-García R, Benavente-Valdepeñas AM. Botulinum toxin: from poison to possible treatment for spasticity in spinal cord injury. Int J Mol Sci. 2021; 22:4886.
Article11. Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol. 2005; 53:3–9.
Article12. Andringa A, van de Port I, van Wegen E, Ket J, Meskers C, Kwakkel G. Effectiveness of botulinum toxin treatment for upper limb spasticity poststroke over different ICF domains: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2019; 100:1703–25.
Article13. Dong Y, Wu T, Hu X, Wang T. Efficacy and safety of botulinum toxin type A for upper limb spasticity after stroke or traumatic brain injury: a systematic review with meta-analysis and trial sequential analysis. Eur J Phys Rehabil Med. 2017; 53:256–67.
Article14. Lui J, Sarai M, Mills PB. Chemodenervation for treatment of limb spasticity following spinal cord injury: a systematic review. Spinal Cord. 2015; 53:252–64.
Article15. Yan X, Lan J, Liu Y, Miao J. Efficacy and safety of botulinum toxin type A in spasticity caused by spinal cord injury: a randomized, controlled trial. Med Sci Monit. 2018; 24:8160–71.
Article16. De Icco R, Perrotta A, Berra E, Allena M, Alfonsi E, Tamburin S, et al. OnabotulinumtoxinA reduces temporal pain processing at spinal level in patients with lower limb spasticity. Toxins. 2019; 11:359.
Article17. Lakra C, Cohen H. A clinical review of the use of Botulinum Toxin type A in managing central neuropathic pain in patients with spinal cord injury. J Spinal Cord Med. 2022; 45:651–5.
Article18. Wissel J. Treatment of spasticity-related pain syndromes. In : Jost WH, editor. Botulinum toxin in painful diseases. Karger Publishers;2003. p. 126–39.19. Taova S. GetData Digitizing Program Code: Description, Testing, Training. 2013.20. Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003; 83:713–21.
Article21. Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018; 23:60–3.
Article22. Sumsuzzman DM, Choi J, Jin Y, Hong Y. Neurocognitive effects of melatonin treatment in healthy adults and individuals with Alzheimer's disease and insomnia: a systematic review and meta-analysis of randomized controlled trials. Neurosci Biobehav Rev. 2021; 127:459–73.
Article23. Higgins JPT, Green S. 7.7.3.3 Obtaining standard deviations from standard errors, confidence intervals, t values and P values for differences in means [Internet]. The Cochrane Collaboration; 2011 [cited 2024 Mar 2]. Available from: https://handbook-5-1.cochrane.org/chapter_7/7_7_3_3_obtaining_standard_deviations_from_standard_errors.htm.24. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–60.
Article25. Sumsuzzman DM, Khan ZA, Choi J, Hong Y. Differential role of melatonin in healthy brain aging: a systematic review and meta-analysis of the SAMP8 model. Aging (Albany NY). 2021; 13:9373–97.
Article26. Sumsuzzman DM, Khan ZA, Choi J, Hong Y. Assessment of functional roles and therapeutic potential of integrin receptors in osteoarthritis: a systematic review and meta-analysis of preclinical studies. Ageing Res Rev. 2022; 81:101729.
Article27. Richardson D, Sheean G, Werring D, Desai M, Edwards S, Greenwood R, et al. Evaluating the role of botulinum toxin in the management of focal hypertonia in adults. J Neurol Neurosurg Psychiatry. 2000; 69:499–506.
Article28. Bernuz B, Genet F, Terrat P, Pradon D, Barbot F, Bussel B, et al. Botulinum toxin effect on voluntary and stretch reflex-related torque produced by the quadriceps: an isokinetic pilot study. Neurorehabil Neural Repair. 2012; 26:542–7.
Article29. Béseler MR, Grao CM, Gil A, Martínez Lozano MD. Walking assessment with instrumented insoles in patients with lower limb spasticity after botulinum toxin infiltration. Neurologia. 2012; 27:519–30.
Article30. Opara J, Hordyńska E, Swoboda A. Effectiveness of botulinum toxin A in the treatment of spasticity of the lower extremities in adults - preliminary report. Ortop Traumatol Rehabil. 2007; 9:277–85.31. Spiegl UJ, Maier D, Gonschorek O, Heyde CE, Bühren V. Antispastic therapy with botulinum toxin type A in patients with traumatic spinal cord lesion. GMS Interdiscip Plast Reconstr Surg DGPW. 2014; 3:Doc14.32. Al-Khodairy AT, Gobelet C, Rossier AB. Has botulinum toxin type A a place in the treatment of spasticity in spinal cord injury patients? Spinal Cord. 1998; 36:854–8.
Article33. Frost G, Finlayson H, Saeidiborojeni S, Lagnau P, Reebye R. Perioperative botulinum toxin injections to enhance surgical outcomes in patients with spasticity: preoperative, intraoperative, and postoperative case reports. Arch Rehabil Res Clin Transl. 2021; 3:100101.
Article34. Gross R, Leboeuf F, Rémy-Néris O, Perrouin-Verbe B. Unstable gait due to spasticity of the rectus femoris: gait analysis and motor nerve block. Ann Phys Rehabil Med. 2012; 55:609–22.
Article35. Htwe O, Hussain RI, Naicker AS. Challenges in managing severe lower limb spasticity associated with bilateral hip joints subluxation. Electron J Gen Med. 2016; 13:165–7.
Article36. Naicker AS, Roohi SA, Chan JL. Botulinum toxin type A for rehabilitation after a spinal cord injury: a case report. J Orthop Surg (Hong Kong). 2009; 17:96–9.
Article37. Richardson D, Edwards S, Sheean GL, Greenwood RJ, Thompson AJ. The effect of botulinum toxin on hand function after incomplete spinal cord injury at the level of C5/6: a case report. Clin Rehabil. 1997; 11:288–92.
Article38. Tang N, Stickevers S, Awan G. Delayed onset of Brown-Sequard syndrome involving upper extremity pain. Pain Pract. 2009; 9:150–1.
Article39. Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn't. BMJ. 1996; 312:71–2.
Article40. Francisco GE, Bandari DS, Bavikatte G, Jost WH, McCusker E, Largent J, et al. High clinician- and patient-reported satisfaction with individualized onabotulinumtoxinA treatment for spasticity across several etiologies from the ASPIRE study. Toxicon X. 2020; 7:100040.
Article41. Andresen SR, Biering-Sørensen F, Hagen EM, Nielsen JF, Bach FW, Finnerup NB. Pain, spasticity and quality of life in individuals with traumatic spinal cord injury in Denmark. Spinal Cord. 2016; 54:973–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Practical Points of Botulinum Toxin Injections for Spasticity of Upper Extremity
- Efficacy and Safety of OnabotulinumtoxinA in Patients With Neurogenic Detrusor Overactivity Caused by Spinal Cord Injury: A Systematic Review and Meta-analysis
- Obturator Nerve Block with Botulinum Toxin Type B for Patient with Adductor Thigh Muscle Spasm: A Case Report
- Botulinum Toxin A Injection into the Subscapularis Muscle to Treat Intractable Hemiplegic Shoulder Pain
- Application of Botulinum Toxin in Pain Management