Endocrinol Metab.
2024 Jun;39(3):425-444. 10.3803/EnM.2023.1802.
Metabolic Reprogramming in Thyroid Cancer
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Chungnam National University Hospital, Daejeon, Korea
- 2Department of Internal Medicine, Chungnam National University College of Medicine, Daejeon, Korea
- 3Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Korea
- KMID: 2556634
- DOI: http://doi.org/10.3803/EnM.2023.1802
Abstract
- Thyroid cancer is a common endocrine malignancy with increasing incidence globally. Although most cases can be treated effectively, some cases are more aggressive and have a higher risk of mortality. Inhibiting RET and BRAF kinases has emerged as a potential therapeutic strategy for the treatment of thyroid cancer, particularly in cases of advanced or aggressive disease. However, the development of resistance mechanisms may limit the efficacy of these kinase inhibitors. Therefore, developing precise strategies to target thyroid cancer cell metabolism and overcome resistance is a critical area of research for advancing thyroid cancer treatment. In the field of cancer therapeutics, researchers have explored combinatorial strategies involving dual metabolic inhibition and metabolic inhibitors in combination with targeted therapy, chemotherapy, and immunotherapy to overcome the challenge of metabolic plasticity. This review highlights the need for new therapeutic approaches for thyroid cancer and discusses promising metabolic inhibitors targeting thyroid cancer. It also discusses the challenges posed by metabolic plasticity in the development of effective strategies for targeting cancer cell metabolism and explores the potential advantages of combined metabolic targeting.
Keyword
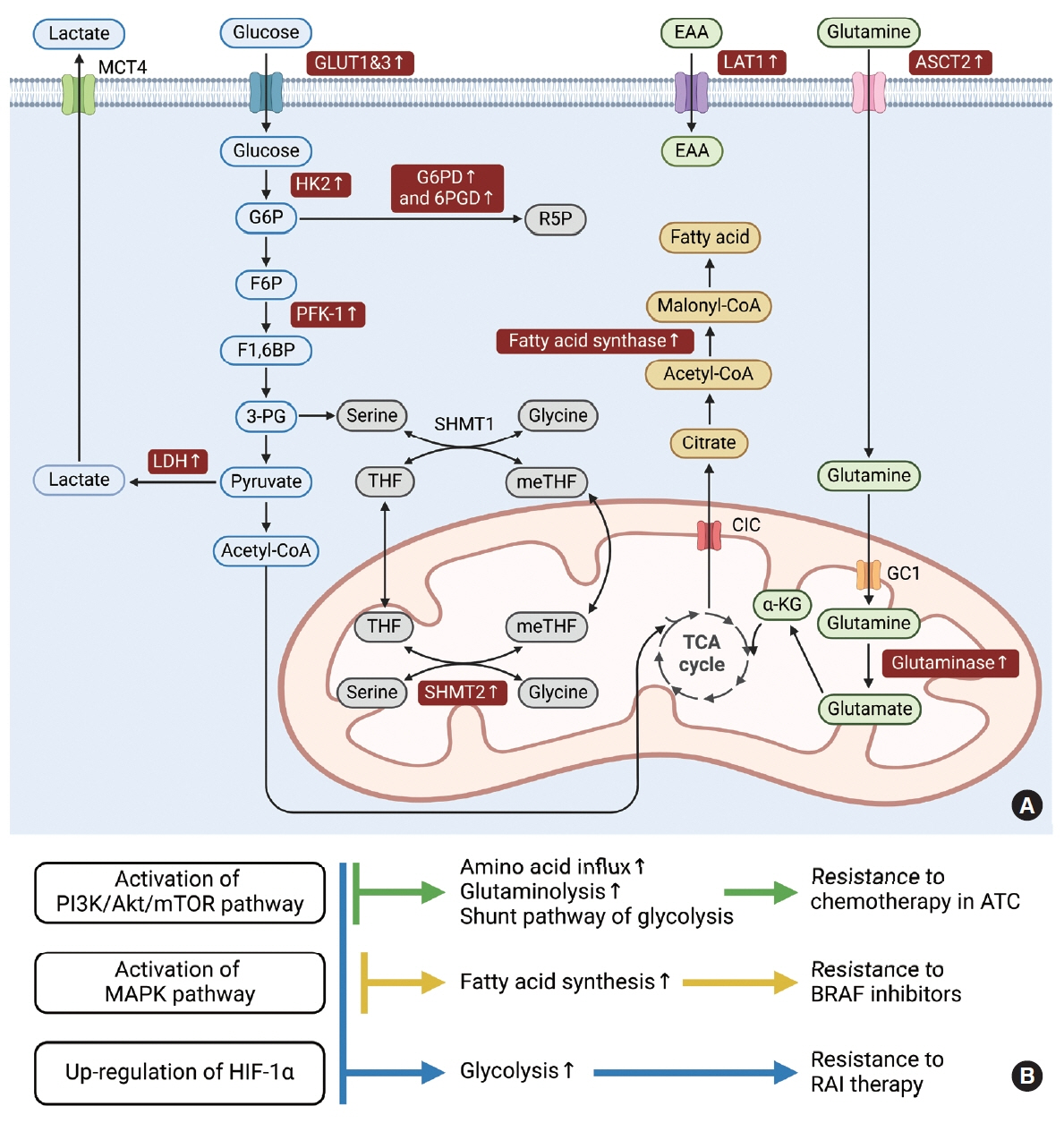
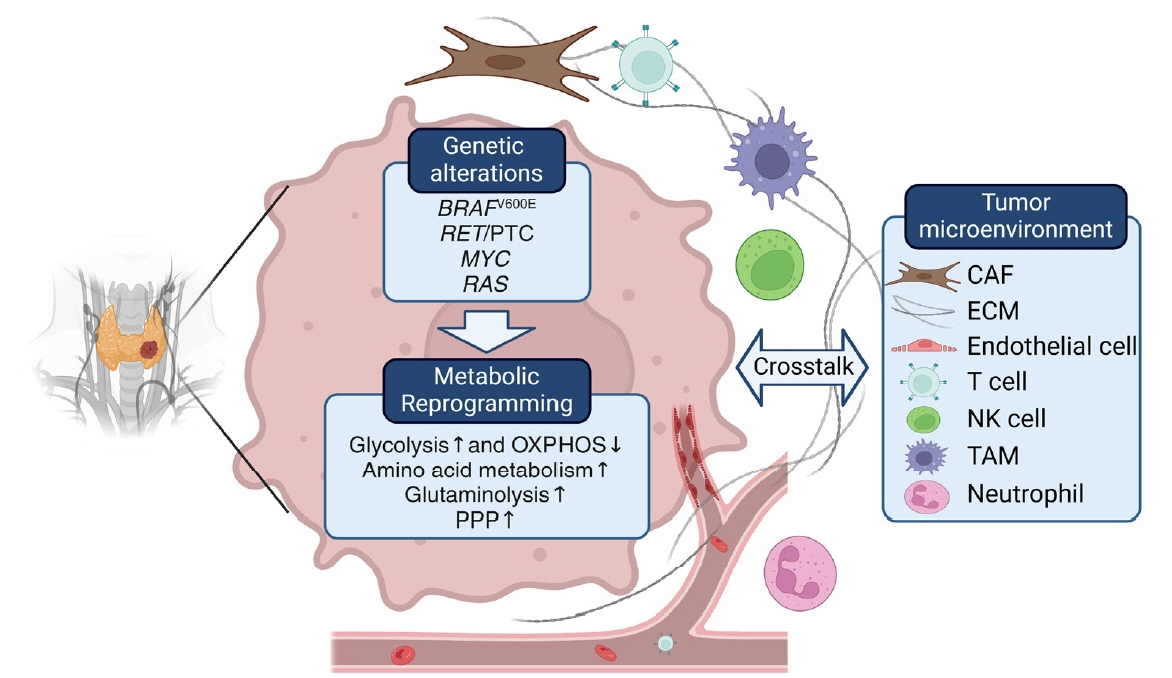
Figure
Reference
-
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–49.
Article2. Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol. 2013; 2013:965212.
Article3. Sanchez-Ares M, Cameselle-Garcia S, Abdulkader-Nallib I, Rodriguez-Carnero G, Beiras-Sarasquete C, Punal-Rodriguez JA, et al. Susceptibility genes and chromosomal regions associated with non-syndromic familial non-medullary thyroid carcinoma: some pathogenetic and diagnostic keys. Front Endocrinol (Lausanne). 2022; 13:829103.4. Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013; 13:184–99.
Article5. Romei C, Elisei R. RET/PTC translocations and clinicopathological features in human papillary thyroid carcinoma. Front Endocrinol (Lausanne). 2012; 3:54.
Article6. Landa I, Cabanillas ME. Genomic alterations in thyroid cancer: biological and clinical insights. Nat Rev Endocrinol. 2024; 20:93–110.
Article7. Cuomo F, Giani C, Cobellis G. The role of the kinase inhibitors in thyroid cancers. Pharmaceutics. 2022; 14:1040.
Article8. Subbiah V, Hu MI, Wirth LJ, Schuler M, Mansfield AS, Curigliano G, et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol. 2021; 9:491–501.
Article9. Wirth LJ, Sherman E, Robinson B, Solomon B, Kang H, Lorch J, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med. 2020; 383:825–35.
Article10. Rosen EY, Goldman DA, Hechtman JF, Benayed R, Schram AM, Cocco E, et al. TRK fusions are enriched in cancers with uncommon histologies and the absence of canonical driver mutations. Clin Cancer Res. 2020; 26:1624–32.
Article11. Hong DS, DuBois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020; 21:531–40.
Article12. Ma Y, Zhang Q, Zhang K, Liang Y, Ren F, Zhang J, et al. NTRK fusions in thyroid cancer: pathology and clinical aspects. Crit Rev Oncol Hematol. 2023; 184:103957.
Article13. Thein KZ, Velcheti V, Mooers BH, Wu J, Subbiah V. Precision therapy for RET-altered cancers with RET inhibitors. Trends Cancer. 2021; 7:1074–88.
Article14. Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005; 12:245–62.
Article15. Kopetz S, Desai J, Chan E, Hecht JR, O’Dwyer PJ, Maru D, et al. Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J Clin Oncol. 2015; 33:4032–8.16. Falchook GS, Millward M, Hong D, Naing A, Piha-Paul S, Waguespack SG, et al. BRAF inhibitor dabrafenib in patients with metastatic BRAF-mutant thyroid cancer. Thyroid. 2015; 25:71–7.
Article17. Crispo F, Notarangelo T, Pietrafesa M, Lettini G, Storto G, Sgambato A, et al. BRAF inhibitors in thyroid cancer: clinical impact, mechanisms of resistance and future perspectives. Cancers (Basel). 2019; 11:1388.
Article18. Subbiah V, Baik C, Kirkwood JM. Clinical development of BRAF plus MEK inhibitor combinations. Trends Cancer. 2020; 6:797–810.
Article19. Zhu Y, Li X, Wang L, Hong X, Yang J. Metabolic reprogramming and crosstalk of cancer-related fibroblasts and immune cells in the tumor microenvironment. Front Endocrinol (Lausanne). 2022; 13:988295.
Article20. Sun HR, Wang S, Yan SC, Zhang Y, Nelson PJ, Jia HL, et al. Therapeutic strategies targeting cancer stem cells and their microenvironment. Front Oncol. 2019; 9:1104.
Article21. Riganti C, Gazzano E, Polimeni M, Aldieri E, Ghigo D. The pentose phosphate pathway: an antioxidant defense and a crossroad in tumor cell fate. Free Radic Biol Med. 2012; 53:421–36.
Article22. Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016; 23:27–47.
Article23. Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016; 41:211–8.
Article24. Jozwiak P, Krzeslak A, Pomorski L, Lipinska A. Expression of hypoxia-related glucose transporters GLUT1 and GLUT3 in benign, malignant and non-neoplastic thyroid lesions. Mol Med Rep. 2012; 6:601–6.
Article25. Nahm JH, Kim HM, Koo JS. Glycolysis-related protein expression in thyroid cancer. Tumour Biol. 2017; 39:1010428317695922.
Article26. Bao L, Xu T, Lu X, Huang P, Pan Z, Ge M. Metabolic reprogramming of thyroid cancer cells and crosstalk in their microenvironment. Front Oncol. 2021; 11:773028.
Article27. Burrows N, Babur M, Resch J, Williams KJ, Brabant G. Hypoxia-inducible factor in thyroid carcinoma. J Thyroid Res. 2011; 2011:762905.
Article28. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133.
Article29. Heydarzadeh S, Moshtaghie AA, Daneshpoor M, Hedayati M. Regulators of glucose uptake in thyroid cancer cell lines. Cell Commun Signal. 2020; 18:83.
Article30. Coelho RG, Cazarin JM, Cavalcanti de Albuquerque JP, de Andrade BM, Carvalho DP. Differential glycolytic profile and Warburg effect in papillary thyroid carcinoma cell lines. Oncol Rep. 2016; 36:3673–81.
Article31. Ricarte-Filho JC, Ryder M, Chitale DA, Rivera M, Heguy A, Ladanyi M, et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009; 69:4885–93.32. Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010; 35:427–33.
Article33. Wei Z, Liu X, Cheng C, Yu W, Yi P. Metabolism of amino acids in cancer. Front Cell Dev Biol. 2021; 8:603837.
Article34. Hafliger P, Graff J, Rubin M, Stooss A, Dettmer MS, Altmann KH, et al. The LAT1 inhibitor JPH203 reduces growth of thyroid carcinoma in a fully immunocompetent mouse model. J Exp Clin Cancer Res. 2018; 37:234.
Article35. Doolittle WK, Park S, Lee SG, Jeong S, Lee G, Ryu D, et al. Non-genomic activation of the AKT-mTOR pathway by the mitochondrial stress response in thyroid cancer. Oncogene. 2022; 41:4893–904.
Article36. Enomoto K, Sato F, Tamagawa S, Gunduz M, Onoda N, Uchino S, et al. A novel therapeutic approach for anaplastic thyroid cancer through inhibition of LAT1. Sci Rep. 2019; 9:14616.37. Davidson CD, Carr FE. Review of pharmacological inhibition of thyroid cancer metabolism. J Cancer Metastasis Treat. 2021; 7:45.
Article38. Yuan J, Guo Y. Targeted therapy for anaplastic thyroid carcinoma: advances and management. Cancers (Basel). 2022; 15:179.
Article39. Anderson NM, Mucka P, Kern JG, Feng H. The emerging role and targetability of the TCA cycle in cancer metabolism. Protein Cell. 2018; 9:216–37.
Article40. Icard P, Coquerel A, Wu Z, Gligorov J, Fuks D, Fournel L, et al. Understanding the central role of citrate in the metabolism of cancer cells and tumors: an update. Int J Mol Sci. 2021; 22:6587.
Article41. Lee S, Rauch J, Kolch W. Targeting MAPK signaling in cancer: mechanisms of drug resistance and sensitivity. Int J Mol Sci. 2020; 21:1102.42. Delgado-Goni T, Miniotis MF, Wantuch S, Parkes HG, Marais R, Workman P, et al. The BRAF inhibitor vemurafenib activates mitochondrial metabolism and inhibits hyperpolarized pyruvate-lactate exchange in BRAF-mutant human melanoma cells. Mol Cancer Ther. 2016; 15:2987–99.43. Nagayama Y, Hamada K. Reprogramming of cellular metabolism and its therapeutic applications in thyroid cancer. Metabolites. 2022; 12:1214.
Article44. Coelho RG, Fortunato RS, Carvalho DP. Metabolic reprogramming in thyroid carcinoma. Front Oncol. 2018; 8:82.
Article45. Yang L, Venneti S, Nagrath D. Glutaminolysis: a hallmark of cancer metabolism. Annu Rev Biomed Eng. 2017; 19:163–94.
Article46. Yoo HC, Yu YC, Sung Y, Han JM. Glutamine reliance in cell metabolism. Exp Mol Med. 2020; 52:1496–516.47. Kim HM, Lee YK, Koo JS. Expression of glutamine metabolism-related proteins in thyroid cancer. Oncotarget. 2016; 7:53628–41.
Article48. Baenke F, Chaneton B, Smith M, Van Den Broek N, Hogan K, Tang H, et al. Resistance to BRAF inhibitors induces glutamine dependency in melanoma cells. Mol Oncol. 2016; 10:73–84.
Article49. Liu CL, Hsu YC, Lee JJ, Chen MJ, Lin CH, Huang SY, et al. Targeting the pentose phosphate pathway increases reactive oxygen species and induces apoptosis in thyroid cancer cells. Mol Cell Endocrinol. 2020; 499:110595.50. Koundouros N, Poulogiannis G. Reprogramming of fatty acid metabolism in cancer. Br J Cancer. 2020; 122:4–22.
Article51. Shafee N, Kaluz S, Ru N, Stanbridge EJ. PI3K/Akt activity has variable cell-specific effects on expression of HIF target genes, CA9 and VEGF, in human cancer cell lines. Cancer Lett. 2009; 282:109–15.52. Agani F, Jiang BH. Oxygen-independent regulation of HIF1: novel involvement of PI3K/AKT/mTOR pathway in cancer. Curr Cancer Drug Targets. 2013; 13:245–51.53. Sharma A, Sinha S, Shrivastava N. Therapeutic targeting hypoxia-inducible factor (HIF-1) in cancer: cutting gordian knot of cancer cell metabolism. Front Genet. 2022; 13:849040.
Article54. Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009; 458:1056–60.
Article55. Zerilli M, Zito G, Martorana A, Pitrone M, Cabibi D, Cappello F, et al. BRAF(V600E) mutation influences hypoxiainducible factor-1alpha expression levels in papillary thyroid cancer. Mod Pathol. 2010; 23:1052–60.56. Grabellus F, Worm K, Schmid KW, Sheu SY. The BRAF V600E mutation in papillary thyroid carcinoma is associated with glucose transporter 1 overexpression. Thyroid. 2012; 22:377–82.
Article57. Song H, Qiu Z, Wang Y, Xi C, Zhang G, Sun Z, et al. HIF-1α/YAP signaling rewrites glucose/iodine metabolism program to promote papillary thyroid cancer progression. Int J Biol Sci. 2023; 19:225–41.58. Kim DW, Hwang JH, Suh JM, Kim H, Song JH, Hwang ES, et al. RET/PTC (rearranged in transformation/papillary thyroid carcinomas) tyrosine kinase phosphorylates and activates phosphoinositide-dependent kinase 1 (PDK1): an alternative phosphatidylinositol 3-kinase-independent pathway to activate PDK1. Mol Endocrinol. 2003; 17:1382–94.59. Osthus RC, Shim H, Kim S, Li Q, Reddy R, Mukherjee M, et al. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J Biol Chem. 2000; 275:21797–800.60. Tambay V, Raymond VA, Bilodeau M. MYC rules: leading glutamine metabolism toward a distinct cancer cell phenotype. Cancers (Basel). 2021; 13:4484.61. Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011; 7:569–80.
Article62. Moura MM, Cavaco BM, Leite V. RAS proto-oncogene in medullary thyroid carcinoma. Endocr Relat Cancer. 2015; 22:R235–52.63. Mukhopadhyay S, Vander Heiden MG, McCormick F. The metabolic landscape of RAS-driven cancers from biology to therapy. Nat Cancer. 2021; 2:271–83.64. Kim YH, Yoon SJ, Kim M, Kim HH, Song YS, Jung JW, et al. Integrative multi-omics analysis reveals different metabolic phenotypes based on molecular characteristics in thyroid cancer. Clin Cancer Res. 2024; 30:883–94.65. Prante O, Maschauer S, Fremont V, Reinfelder J, Stoehr R, Szkudlinski M, et al. Regulation of uptake of 18F-FDG by a follicular human thyroid cancer cell line with mutationactivated K-ras. J Nucl Med. 2009; 50:1364–70.66. Bernfeld E, Foster DA. Glutamine as an essential amino acid for KRas-driven cancer cells. Trends Endocrinol Metab. 2019; 30:357–68.67. Martin MJ, Eberlein C, Taylor M, Ashton S, Robinson D, Cross D. Inhibition of oxidative phosphorylation suppresses the development of Osimertinib resistance in a preclinical model of EGFR-driven lung adenocarcinoma. Oncotarget. 2016; 7:86313–25.68. Haq R, Shoag J, Andreu-Perez P, Yokoyama S, Edelman H, Rowe GC, et al. Oncogenic BRAF regulates oxidative metabolism via PGC1α and MITF. Cancer Cell. 2013; 23:302–15.69. Lee HJ, Zhuang G, Cao Y, Du P, Kim HJ, Settleman J. Drug resistance via feedback activation of Stat3 in oncogene-addicted cancer cells. Cancer Cell. 2014; 26:207–21.70. Lee M, Hirpara JL, Eu JQ, Sethi G, Wang L, Goh BC, et al. Targeting STAT3 and oxidative phosphorylation in oncogene-addicted tumors. Redox Biol. 2019; 25:101073.71. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014; 384:319–28.72. Brose MS, Cabanillas ME, Cohen EE, Wirth LJ, Riehl T, Yue H, et al. Vemurafenib in patients with BRAF(V600E)- positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: a non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016; 17:1272–82.73. Yan X, Tian R, Sun J, Zhao Y, Liu B, Su J, et al. Sorafenib-induced autophagy promotes glycolysis by upregulating the p62/HDAC6/HSP90 axis in hepatocellular carcinoma cells. Front Pharmacol. 2022; 12:788667.74. Prieto-Dominguez N, Ordonez R, Fernandez A, GarciaPalomo A, Muntane J, Gonzalez-Gallego J, et al. Modulation of autophagy by sorafenib: effects on treatment response. Front Pharmacol. 2016; 7:151.75. Sandulache VC, Skinner HD, Wang Y, Chen Y, Dodge CT, Ow TJ, et al. Glycolytic inhibition alters anaplastic thyroid carcinoma tumor metabolism and improves response to conventional chemotherapy and radiation. Mol Cancer Ther. 2012; 11:1373–80.76. Kc YB, Jeoung NH. Anti-cancer effect of dichloroacetate in human anaplastic thyroid cancer cell line, 8505-C. In: 2015 International Conference on Diabetes and Metabolism; 2015 Oct 15-17; Jeju, Korea. Seoul: Korean Diabetes Association; 2015. p. 177.77. Jingtai Z, Linfei H, Yuyang Q, Ning K, Xinwei Y, Xin W, et al. Targeting Aurora-A inhibits tumor progression and sensitizes thyroid carcinoma to Sorafenib by decreasing PFKFB3-mediated glycolysis. Cell Death Dis. 2023; 14:224.78. Niehr F, von Euw E, Attar N, Guo D, Matsunaga D, Sazegar H, et al. Combination therapy with vemurafenib (PLX4032/RG7204) and metformin in melanoma cell lines with distinct driver mutations. J Transl Med. 2011; 9:76.79. Siddharth S, Kuppusamy P, Wu Q, Nagalingam A, Saxena NK, Sharma D. Metformin enhances the anti-cancer efficacy of sorafenib via suppressing MAPK/ERK/Stat3 axis in hepatocellular carcinoma. Int J Mol Sci. 2022; 23:8083.80. Yuan P, Ito K, Perez-Lorenzo R, Del Guzzo C, Lee JH, Shen CH, et al. Phenformin enhances the therapeutic benefit of BRAF(V600E) inhibition in melanoma. Proc Natl Acad Sci U S A. 2013; 110:18226–31.81. Xiao Y, Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther. 2021; 221:107753.82. Li Z, Sun C, Qin Z. Metabolic reprogramming of cancerassociated fibroblasts and its effect on cancer cell reprogramming. Theranostics. 2021; 11:8322–36.83. Wilde L, Roche M, Domingo-Vidal M, Tanson K, Philp N, Curry J, et al. Metabolic coupling and the Reverse Warburg Effect in cancer: implications for novel biomarker and anticancer agent development. Semin Oncol. 2017; 44:198–203.84. Curry JM, Tassone P, Cotzia P, Sprandio J, Luginbuhl A, Cognetti DM, et al. Multicompartment metabolism in papillary thyroid cancer. Laryngoscope. 2016; 126:2410–8.85. Claiborne MD, Leone R. Differential glutamine metabolism in the tumor microenvironment: studies in diversity and heterogeneity: a mini-review. Front Oncol. 2022; 12:1011191.86. Elia I, Haigis MC. Metabolites and the tumour microenvironment: from cellular mechanisms to systemic metabolism. Nat Metab. 2021; 3:21–32.87. Fozzatti L, Alamino VA, Park S, Giusiano L, Volpini X, Zhao L, et al. Interplay of fibroblasts with anaplastic tumor cells promotes follicular thyroid cancer progression. Sci Rep. 2019; 9:8028.88. Lidonnici J, Santoro MM, Oberkersch RE. Cancer-induced metabolic rewiring of tumor endothelial cells. Cancers (Basel). 2022; 14:2735.89. Cantelmo AR, Conradi LC, Brajic A, Goveia J, Kalucka J, Pircher A, et al. Inhibition of the glycolytic activator PFKFB3 in endothelium induces tumor vessel normalization, impairs metastasis, and improves chemotherapy. Cancer Cell. 2016; 30:968–85.90. Carmona-Fontaine C, Deforet M, Akkari L, Thompson CB, Joyce JA, Xavier JB. Metabolic origins of spatial organization in the tumor microenvironment. Proc Natl Acad Sci U S A. 2017; 114:2934–9.91. Schoors S, Bruning U, Missiaen R, Queiroz KC, Borgers G, Elia I, et al. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature. 2015; 520:192–7.92. Salemme V, Centonze G, Cavallo F, Defilippi P, Conti L. The crosstalk between tumor cells and the immune microenvironment in breast cancer: implications for immunotherapy. Front Oncol. 2021; 11:610303.93. Xia L, Oyang L, Lin J, Tan S, Han Y, Wu N, et al. The cancer metabolic reprogramming and immune response. Mol Cancer. 2021; 20:28.94. van der Windt GJ, Pearce EL. Metabolic switching and fuzel choice during T-cell differentiation and memory development. Immunol Rev. 2012; 249:27–42.95. Cuyas E, Verdura S, Martin-Castillo B, Alarcon T, Lupu R, Bosch-Barrera J, et al. Tumor cell-intrinsic immunometabolism and precision nutrition in cancer immunotherapy. Cancers (Basel). 2020; 12:1757.96. Yang Y, Li C, Liu T, Dai X, Bazhin AV. Myeloid-derived suppressor cells in tumors: from mechanisms to antigen specificity and microenvironmental regulation. Front Immunol. 2020; 11:1371.97. Tie Y, Tang F, Wei YQ, Wei XW. Immunosuppressive cells in cancer: mechanisms and potential therapeutic targets. J Hematol Oncol. 2022; 15:61.98. Terren I, Orrantia A, Vitalle J, Zenarruzabeitia O, Borrego F. NK cell metabolism and tumor microenvironment. Front Immunol. 2019; 10:2278.99. Park A, Yang Y, Lee Y, Kim MS, Park YJ, Jung H, et al. Indoleamine-2,3-dioxygenase in thyroid cancer cells suppresses natural killer cell function by inhibiting NKG2D and NKp46 expression via STAT signaling pathways. J Clin Med. 2019; 8:842.100. Park A, Lee Y, Kim MS, Kang YJ, Park YJ, Jung H, et al. Prostaglandin E2 secreted by thyroid cancer cells contributes to immune escape through the suppression of natural killer (NK) cell cytotoxicity and NK cell differentiation. Front Immunol. 2018; 9:1859.101. Rice CM, Davies LC, Subleski JJ, Maio N, Gonzalez-Cotto M, Andrews C, et al. Tumour-elicited neutrophils engage mitochondrial metabolism to circumvent nutrient limitations and maintain immune suppression. Nat Commun. 2018; 9:5099.102. Arts RJ, Plantinga TS, Tuit S, Ulas T, Heinhuis B, Tesselaar M, et al. Transcriptional and metabolic reprogramming induce an inflammatory phenotype in non-medullary thyroid carcinoma-induced macrophages. Oncoimmunology. 2016; 5:e1229725.103. Rabold K, Aschenbrenner A, Thiele C, Boahen CK, Schiltmans A, Smit JW, et al. Enhanced lipid biosynthesis in human tumor-induced macrophages contributes to their protumoral characteristics. J Immunother Cancer. 2020; 8:e000638.104. Caillou B, Talbot M, Weyemi U, Pioche-Durieu C, Al Ghuzlan A, Bidart JM, et al. Tumor-associated macrophages (TAMs) form an interconnected cellular supportive network in anaplastic thyroid carcinoma. PLoS One. 2011; 6:e22567.105. Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016; 39:98–106.106. Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018; 18:153–67.107. Patsoukis N, Bardhan K, Chatterjee P, Sari D, Liu B, Bell LN, et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun. 2015; 6:6692.108. Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015; 162:1229–41.109. Chintakuntlawar AV, Rumilla KM, Smith CY, Jenkins SM, Foote RL, Kasperbauer JL, et al. Expression of PD-1 and PD-L1 in anaplastic thyroid cancer patients treated with multimodal therapy: results from a retrospective study. J Clin Endocrinol Metab. 2017; 102:1943–50.
Article110. Ahn S, Kim TH, Kim SW, Ki CS, Jang HW, Kim JS, et al. Comprehensive screening for PD-L1 expression in thyroid cancer. Endocr Relat Cancer. 2017; 24:97–106.
Article111. Giannini R, Moretti S, Ugolini C, Macerola E, Menicali E, Nucci N, et al. Immune profiling of thyroid carcinomas suggests the existence of two major phenotypes: an ATClike and a PDTC-like. J Clin Endocrinol Metab. 2019; 104:3557–75.112. Garcia-Alvarez A, Hernando J, Carmona-Alonso A, Capdevila J. What is the status of immunotherapy in thyroid neoplasms? Front Endocrinol (Lausanne). 2022; 13:929091.113. Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and antiCTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018; 8:86.114. Liu T, Han C, Wang S, Fang P, Ma Z, Xu L, et al. Cancerassociated fibroblasts: an emerging target of anti-cancer immunotherapy. J Hematol Oncol. 2019; 12:86.
Article115. Palsson-McDermott EM, Dyck L, Zaslona Z, Menon D, McGettrick AF, Mills KH, et al. Pyruvate kinase M2 is required for the expression of the immune checkpoint PD-L1 in immune cells and tumors. Front Immunol. 2017; 8:1300.116. Rotondi M, Coperchini F, Pignatti P, Magri F, Chiovato L. Metformin reverts the secretion of CXCL8 induced by TNF-α in primary cultures of human thyroid cells: an additional indirect anti-tumor effect of the drug. J Clin Endocrinol Metab. 2015; 100:E427–32.117. Yu Y, Feng C, Kuang J, Guo L, Guan H. Metformin exerts an antitumoral effect on papillary thyroid cancer cells through altered cell energy metabolism and sensitized by BACH1 depletion. Endocrine. 2022; 76:116–31.118. Cho SW, Yi KH, Han SK, Sun HJ, Kim YA, Oh BC, et al. Therapeutic potential of metformin in papillary thyroid cancer in vitro and in vivo. Mol Cell Endocrinol. 2014; 393:24–9.119. Thakur S, Daley B, Gaskins K, Vasko VV, Boufraqech M, Patel D, et al. Metformin targets mitochondrial glycerophosphate dehydrogenase to control rate of oxidative phosphorylation and growth of thyroid cancer in vitro and in vivo. Clin Cancer Res. 2018; 24:4030–43.120. Shen CT, Wei WJ, Qiu ZL, Song HJ, Zhang XY, Sun ZK, et al. Metformin reduces glycometabolism of papillary thyroid carcinoma in vitro and in vivo. J Mol Endocrinol. 2017; 58:15–23.121. He Y, Cao L, Wang L, Liu L, Huang Y, Gong X. Metformin inhibits proliferation of human thyroid cancer TPC-1 cells by decreasing LRP2 to suppress the JNK pathway. Onco Targets Ther. 2020; 13:45–50.122. Ye J, Qi L, Chen K, Li R, Song S, Zhou C, et al. Metformin induces TPC-1 cell apoptosis through endoplasmic reticulum stress-associated pathways in vitro and in vivo. Int J Oncol. 2019; 55:331–9.123. Nozhat Z, Zarkesh M, Baldini E, Mohammadi-Yeganeh S, Azizi F, Hedayati M. Antineoplastic activity of an old natural antidiabetic biguanide on the human thyroid carcinoma cell line. Anticancer Agents Med Chem. 2022; 22:713–20.124. Park S, Willingham MC, Qi J, Cheng SY. Metformin and JQ1 synergistically inhibit obesity-activated thyroid cancer. Endocr Relat Cancer. 2018; 25:865–77.125. Shin HS, Sun HJ, Whang YM, Park YJ, Park DJ, Cho SW. Metformin reduces thyroid cancer tumor growth in the metastatic niche of bone by inhibiting osteoblastic RANKL productions. Thyroid. 2021; 31:760–71.
Article126. Coperchini F, Croce L, Denegri M, Awwad O, Ngnitejeu ST, Magri F, et al. The anti-cancer effects of phenformin in thyroid cancer cell lines and in normal thyrocytes. Oncotarget. 2019; 10:6432–43.127. Yu Y, Yu X, Fan C, Wang H, Wang R, Feng C, et al. Targeting glutaminase-mediated glutamine dependence in papillary thyroid cancer. J Mol Med (Berl). 2018; 96:777–90.
Article128. Wang SY, Wei YH, Shieh DB, Lin LL, Cheng SP, Wang PW, et al. 2-Deoxy-d-glucose can complement doxorubicin and sorafenib to suppress the growth of papillary thyroid carcinoma cells. PLoS One. 2015; 10:e0130959.
Article129. Bikas A, Jensen K, Patel A, Costello J Jr, McDaniel D, Klubo-Gwiezdzinska J, et al. Glucose-deprivation increases thyroid cancer cells sensitivity to metformin. Endocr Relat Cancer. 2015; 22:919–32.
Article130. Zhao B, Aggarwal A, Marshall JA, Barletta JA, Kijewski MF, Lorch JH, et al. Glycolytic inhibition with 3-bromopyruvate suppresses tumor growth and improves survival in a murine model of anaplastic thyroid cancer. Surgery. 2022; 171:227–34.
Article131. Dima M, Miller KA, Antico-Arciuch VG, Di Cristofano A. Establishment and characterization of cell lines from a novel mouse model of poorly differentiated thyroid carcinoma: powerful tools for basic and preclinical research. Thyroid. 2011; 21:1001–7.
Article132. Ghavami G, Kiasari RE, Pakzad F, Sardari S. Effect of metformin alone and in combination with etoposide and epirubicin on proliferation, apoptosis, necrosis, and migration of BCPAP and SW cells as thyroid cancer cell lines. Res Pharm Sci. 2023; 18:185–201.
Article133. Ozdemir Kutbay N, Biray Avci C, Sarer Yurekli B, Caliskan Kurt C, Shademan B, Gunduz C, et al. Effects of metformin and pioglitazone combination on apoptosis and AMPK/ mTOR signaling pathway in human anaplastic thyroid cancer cells. J Biochem Mol Toxicol. 2020; 34:e22547.
Article134. Chen G, Xu S, Renko K, Derwahl M. Metformin inhibits growth of thyroid carcinoma cells, suppresses self-renewal of derived cancer stem cells, and potentiates the effect of chemotherapeutic agents. J Clin Endocrinol Metab. 2012; 97:E510–20.
Article135. Durai L, Ravindran S, Arvind K, Karunagaran D, Vijayalakshmi R. Synergistic effect of metformin and vemurufenib (PLX4032) as a molecular targeted therapy in anaplastic thyroid cancer: an in vitro study. Mol Biol Rep. 2021; 48:7443–56.
Article136. Hanly EK, Bednarczyk RB, Tuli NY, Moscatello AL, Halicka HD, Li J, et al. mTOR inhibitors sensitize thyroid cancer cells to cytotoxic effect of vemurafenib. Oncotarget. 2015; 6:39702–13.
Article137. Chen G, Nicula D, Renko K, Derwahl M. Synergistic antiproliferative effect of metformin and sorafenib on growth of anaplastic thyroid cancer cells and their stem cells. Oncol Rep. 2015; 33:1994–2000.
Article138. Kheder S, Sisley K, Hadad S, Balasubramanian SP. Effects of prolonged exposure to low dose metformin in thyroid cancer cell lines. J Cancer. 2017; 8:1053–61.
Article139. Chen Z, Lin J, Feng S, Chen X, Huang H, Wang C, et al. SIRT4 inhibits the proliferation, migration, and invasion abilities of thyroid cancer cells by inhibiting glutamine metabolism. Onco Targets Ther. 2019; 12:2397–408.140. Patel D, King T, Kebebew E, Nilubol N, Boufraqech M. SAT-568 Glutamine metabolism is a new potential therapeutic target in aggressive thyroid cancer. J Endocr Soc. 2019; 3(Supplement 1):SAT–568.
Article141. Raez LE, Papadopoulos K, Ricart AD, Chiorean EG, Dipaola RS, Stein MN, et al. A phase I dose-escalation trial of 2-deoxy-D-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2013; 71:523–30.
Article142. Stein M, Lin H, Jeyamohan C, Dvorzhinski D, Gounder M, Bray K, et al. Targeting tumor metabolism with 2-deoxyglucose in patients with castrate-resistant prostate cancer and advanced malignancies. Prostate. 2010; 70:1388–94.
Article143. Shi L, Pan H, Liu Z, Xie J, Han W. Roles of PFKFB3 in cancer. Signal Transduct Target Ther. 2017; 2:17044.
Article144. Icard P, Loi M, Wu Z, Ginguay A, Lincet H, Robin E, et al. Metabolic strategies for inhibiting cancer development. Adv Nutr. 2021; 12:1461–80.
Article145. Liu X, Olszewski K, Zhang Y, Lim EW, Shi J, Zhang X, et al. Cystine transporter regulation of pentose phosphate pathway dependency and disulfide stress exposes a targetable metabolic vulnerability in cancer. Nat Cell Biol. 2020; 22:476–86.
Article146. Han B, Cui H, Kang L, Zhang X, Jin Z, Lu L, et al. Metformin inhibits thyroid cancer cell growth, migration, and EMT through the mTOR pathway. Tumour Biol. 2015; 36:6295–304.
Article147. Xiao Q, Xiao J, Liu J, Liu J, Shu G, Yin G. Metformin suppresses the growth of colorectal cancer by targeting INHBA to inhibit TGF-β/PI3K/AKT signaling transduction. Cell Death Dis. 2022; 13:202.
Article148. Garcia Rubino ME, Carrillo E, Ruiz Alcala G, DominguezMartin A, A Marchal J, Boulaiz H. Phenformin as an anticancer agent: challenges and prospects. Int J Mol Sci. 2019; 20:3316.
Article149. Morale MG, Tamura RE, Rubio IG. Metformin and cancer hallmarks: molecular mechanisms in thyroid, prostate and head and neck cancer models. Biomolecules. 2022; 12:357.
Article150. Garcia-Saenz M, Lobaton-Ginsberg M, Ferreira-Hermosillo A. Metformin in differentiated thyroid cancer: molecular pathways and its clinical implications. Biomolecules. 2022; 12:574.
Article151. Zhao J, Zhang Q, Yang Y, Yao J, Liao L, Dong J. High prevalence of thyroid carcinoma in patients with insulin resistance: a meta-analysis of case-control studies. Aging (Albany NY). 2021; 13:22232–41.
Article152. Tseng CH, Tseng CP, Chong CK, Huang TP, Song YM, Chou CW, et al. Increasing incidence of diagnosed type 2 diabetes in Taiwan: analysis of data from a national cohort. Diabetologia. 2006; 49:1755–60.
Article153. Cho YY, Kang MJ, Kim SK, Jung JH, Hahm JR, Kim TH, et al. Protective effect of metformin against thyroid cancer development: a population-based study in Korea. Thyroid. 2018; 28:864–70.
Article154. Becker C, Jick SS, Meier CR, Bodmer M. No evidence for a decreased risk of thyroid cancer in association with use of metformin or other antidiabetic drugs: a case-control study. BMC Cancer. 2015; 15:719.
Article155. Yap TA, Daver N, Mahendra M, Zhang J, Kamiya-Matsuoka C, Meric-Bernstam F, et al. Complex I inhibitor of oxidative phosphorylation in advanced solid tumors and acute myeloid leukemia: phase I trials. Nat Med. 2023; 29:115–26.
Article156. Chen D, Barsoumian HB, Fischer G, Yang L, Verma V, Younes AI, et al. Combination treatment with radiotherapy and a novel oxidative phosphorylation inhibitor overcomes PD-1 resistance and enhances antitumor immunity. J Immunother Cancer. 2020; 8:e000289.
Article157. Hassanein M, Qian J, Hoeksema MD, Wang J, Jacobovitz M, Ji X, et al. Targeting SLC1a5-mediated glutamine dependence in non-small cell lung cancer. Int J Cancer. 2015; 137:1587–97.158. Xiang Y, Stine ZE, Xia J, Lu Y, O’Connor RS, Altman BJ, et al. Targeted inhibition of tumor-specific glutaminase diminishes cell-autonomous tumorigenesis. J Clin Invest. 2015; 125:2293–306.
Article159. Boysen G, Jamshidi-Parsian A, Davis MA, Siegel ER, Simecka CM, Kore RA, et al. Glutaminase inhibitor CB-839 increases radiation sensitivity of lung tumor cells and human lung tumor xenografts in mice. Int J Radiat Biol. 2019; 95:436–42.
Article160. de la Cruz Lopez KG, Toledo Guzman ME, Sanchez EO, Garcia Carranca A. mTORC1 as a regulator of mitochondrial functions and a therapeutic target in cancer. Front Oncol. 2019; 9:1373.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Understanding of Cancer Cell Metabolism and Thyroid Cancer
- Development of Metabolic Synthetic Lethality and Its Implications for Thyroid Cancer
- Targeting Lipid Metabolic Reprogramming as Anticancer Therapeutics
- Oncogene-Driven Metabolic Alterations in Cancer
- Metabolic Signaling to Epigenetic Alterations in Cancer