Int J Stem Cells.
2024 May;17(2):120-129. 10.15283/ijsc24044.
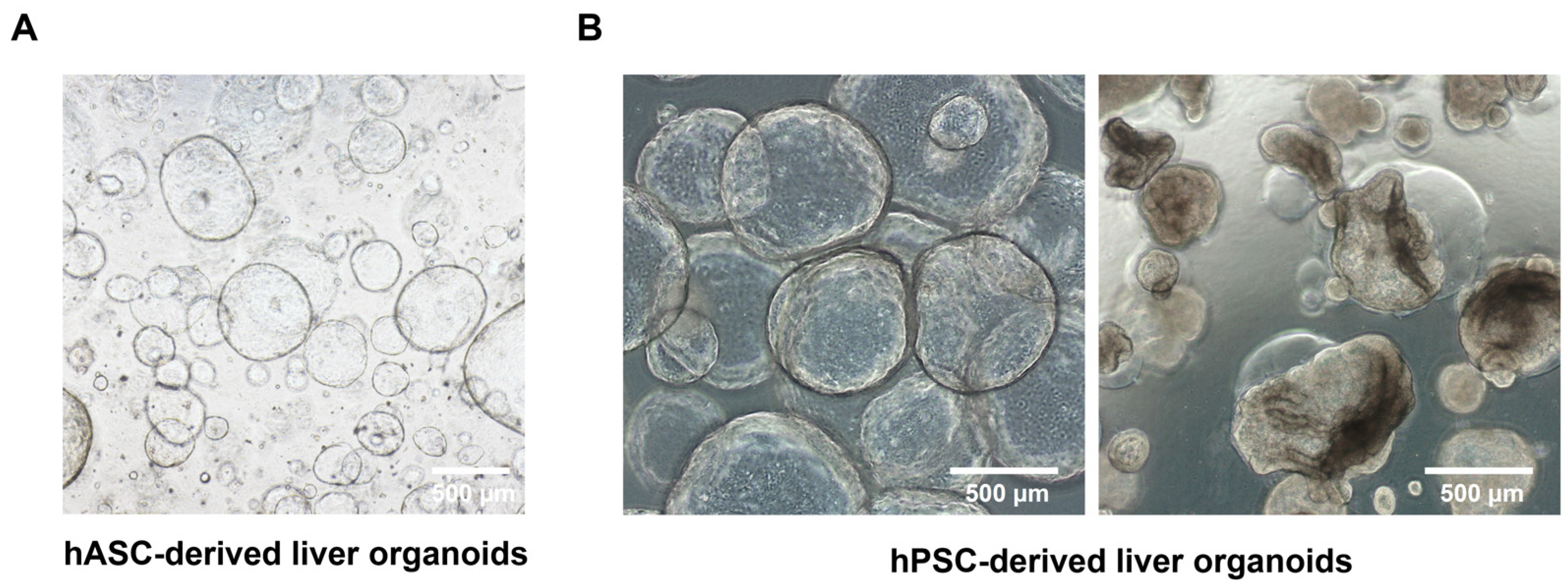
Guidelines for Manufacturing and Application of Organoids: Liver
- Affiliations
-
- 1Stem Cell Convergence Research Center, Korea Research Institute of Bioscience and Biotechnology (KRIBB), Daejeon, Korea
- 2Department of Surgery, Hanyang University College of Medicine, Seoul, Korea
- 3Research Institute of Regenerative Medicine and Stem Cells, Hanyang University, Seoul, Korea
- 4Department of Predictive Toxicology, Korea Institute of Toxicology, Daejeon, Korea
- 5Organoid Standards Initiative
- 6Biometrology Group, Korea Research Institute of Standards and Science (KRISS), Daejeon, Korea
- 7CellArtgen Inc., Seoul, Korea
- 8Department of HY-KIST Bio-convergence, Hanyang University, Seoul, Korea
- 9Department of Biophysics, Sungkyunkwan University, Suwon, Korea
- 10Institute of Quantum Biophysics, Sungkyunkwan University, Suwon, Korea
- 11Department of Functional Genomics, Korea University of Science & Technology (UST), Daejeon, Korea
- KMID: 2556231
- DOI: http://doi.org/10.15283/ijsc24044
Abstract
- Recent amendments to regulatory frameworks have placed a greater emphasis on the utilization of in vitro testing platforms for preclinical drug evaluations and toxicity assessments. This requires advanced tissue models capable of accurately replicating liver functions for drug efficacy and toxicity predictions. Liver organoids, derived from human cell sources, offer promise as a reliable platform for drug evaluation. However, there is a lack of standardized quality evaluation methods, which hinders their regulatory acceptance. This paper proposes comprehensive quality standards tailored for liver organoids, addressing cell source validation, organoid generation, and functional assessment. These guidelines aim to enhance reproducibility and accuracy in toxicity testing, thereby accelerating the adoption of organoids as a reliable alternative or complementary tool to animal testing in drug development. The quality standards include criteria for size, cellular composition, gene expression, and functional assays, thus ensuring a robust hepatotoxicity testing platform.
Figure
Cited by 1 articles
-
Standards for Organoids
Sun-Ju Ahn
Int J Stem Cells. 2024;17(2):99-101. doi: 10.15283/ijsc24043.
Reference
-
References
1. Han JJ. 2023; FDA Modernization Act 2.0 allows for alternatives to animal testing. Artif Organs. 47:449–450. DOI: 10.1111/aor.14503. PMID: 36762462.2. Berggren E, Worth AP. 2023; Towards a future regulatory framework for chemicals in the European Union - Chemicals 2.0. Regul Toxicol Pharmacol. 142:105431. DOI: 10.1016/j.yrtph.2023.105431. PMID: 37315707. PMCID: PMC10390824.
Article3. Olson H, Betton G, Robinson D, et al. 2000; Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul Toxicol Pharmacol. 32:56–67. DOI: 10.1006/rtph.2000.1399. PMID: 11029269.
Article4. Baran SW, Brown PC, Baudy AR, et al. 2022; Perspectives on the evaluation and adoption of complex in vitro models in drug development: workshop with the FDA and the pharmaceutical industry (IQ MPS Affiliate). ALTEX. 39:297–314. DOI: 10.14573/altex.2112203. PMID: 35064273.5. Moon HR, Surianarayanan N, Singh T, Han B. 2023; Microphysiological systems as reliable drug discovery and evaluation tools: evolution from innovation to maturity. Biomicrofluidics. 17:061504. DOI: 10.1063/5.0179444. PMID: 38162229.
Article6. Zhao Z, Chen X, Dowbaj AM, et al. 2022; Organoids. Nat Rev Methods Primers. 2:94. DOI: 10.1038/s43586-022-00174-y. PMID: 37325195. PMCID: PMC10270325.
Article7. Reuben A, Koch DG, Lee WM. 2010; Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 52:2065–2076. DOI: 10.1002/hep.23937. PMID: 20949552. PMCID: PMC3992250.
Article8. Mun SJ, Ryu JS, Lee MO, et al. 2019; Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J Hepatol. 71:970–985. DOI: 10.1016/j.jhep.2019.06.030. PMID: 31299272.
Article9. Osonoi S, Takebe T. 2024; Organoid-guided precision hepatology for metabolic liver disease. J Hepatol. 80:805–821. DOI: 10.1016/j.jhep.2024.01.002. PMID: 38237864.
Article10. Zhang CJ, Meyer SR, O'Meara MJ, et al. 2023; A human liver organoid screening platform for DILI risk prediction. J Hepatol. 78:998–1006. DOI: 10.1016/j.jhep.2023.01.019. PMID: 36738840.
Article11. Brooks A, Liang X, Zhang Y, et al. 2021; Liver organoid as a 3D in vitro model for drug validation and toxicity assessment. Pharmacol Res. 169:105608. DOI: 10.1016/j.phrs.2021.105608. PMID: 33852961.12. Kim H, Im I, Jeon JS, et al. 2022; Development of human pluripotent stem cell-derived hepatic organoids as an alternative model for drug safety assessment. Biomaterials. 286:121575. DOI: 10.1016/j.biomaterials.2022.121575. PMID: 35598335.
Article13. Takebe T, Sekine K, Enomura M, et al. 2013; Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 499:481–484. DOI: 10.1038/nature12271. PMID: 23823721.
Article14. Shinozawa T, Kimura M, Cai Y, et al. 2021; High-fidelity drug-induced liver injury screen using human pluripotent stem cell-derived organoids. Gastroenterology. 160:831–846.e10. DOI: 10.1053/j.gastro.2020.10.002. PMID: 33039464. PMCID: PMC7878295.
Article15. Marsee A, Roos FJM, Verstegen MMA, et al. 2021; Building consensus on definition and nomenclature of hepatic, pancreatic, and biliary organoids. Cell Stem Cell. 28:816–832. DOI: 10.1016/j.stem.2021.04.005. PMID: 33961769.
Article16. Huch M, Gehart H, van Boxtel R, et al. 2015; Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 160:299–312. DOI: 10.1016/j.cell.2014.11.050. PMID: 25533785. PMCID: PMC4313365.
Article17. Kim HJ, Kim G, Chi KY, Kim JH. 2023; In vitro generation of luminal vasculature in liver organoids: from basic vascular biology to vascularized hepatic organoids. Int J Stem Cells. 16:1–15. DOI: 10.15283/ijsc22154. PMID: 36310029. PMCID: PMC9978835.
Article18. Guan Y, Enejder A, Wang M, et al. 2021; A human multi-lineage hepatic organoid model for liver fibrosis. Nat Commun. 12:6138. DOI: 10.1038/s41467-021-26410-9. PMID: 34686668. PMCID: PMC8536785.
Article19. Jensen KB, Little MH. 2023; Organoids are not organs: sources of variation and misinformation in organoid biology. Stem Cell Reports. 18:1255–1270. DOI: 10.1016/j.stemcr.2023.05.009. PMID: 37315519. PMCID: PMC10277837.
Article20. Harrison SP, Siller R, Tanaka Y, et al. 2023; Scalable production of tissue-like vascularized liver organoids from human PSCs. Exp Mol Med. 55:2005–2024. DOI: 10.1038/s12276-023-01074-1. PMID: 37653039. PMCID: PMC10545717.
Article21. Salas-Silva S, Kim Y, Kim TH, et al. 2023; Human chemically-derived hepatic progenitors (hCdHs) as a source of liver organoid generation: application in regenerative medicine, disease modeling, and toxicology testing. Biomaterials. 303:122360. DOI: 10.1016/j.biomaterials.2023.122360. PMID: 38465578.
Article22. Takahashi K, Tanabe K, Ohnuki M, et al. 2007; Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 131:861–872. DOI: 10.1016/j.cell.2007.11.019. PMID: 18035408.
Article23. Kim JH, Mun SJ, Kim JH, Son MJ, Kim SY. 2023; Integrative analysis of single-cell RNA-seq and ATAC-seq reveals heterogeneity of induced pluripotent stem cell-derived hepatic organoids. iScience. 26:107675. DOI: 10.1016/j.isci.2023.107675. PMID: 37680467. PMCID: PMC10481365.
Article24. Ouchi R, Togo S, Kimura M, et al. 2019; Modeling steatohepatitis in humans with pluripotent stem cell-derived organoids. Cell Metab. 30:374–384.e6. DOI: 10.1016/j.cmet.2019.05.007. PMID: 31155493. PMCID: PMC6687537.
Article25. Lee J, Gil D, Park H, et al. 2023; A multicellular liver organoid model for investigating hepatitis C virus infection and nonalcoholic fatty liver disease progression. Hepatology. https://doi.org/10.1097/HEP.0000000000000683 [Epub ahead of print]. DOI: 10.1097/HEP.0000000000000683.
Article26. Pek NMQ, Liu KJ, Nichane M, Ang LT. 2021; Controversies surrounding the origin of hepatocytes in adult livers and the in vitro generation or propagation of hepatocytes. Cell Mol Gastroenterol Hepatol. 11:273–290. DOI: 10.1016/j.jcmgh.2020.09.016. PMID: 32992051. PMCID: PMC7695885.
Article27. Sohlenius-Sternbeck AK. 2006; Determination of the hepatocellularity number for human, dog, rabbit, rat and mouse livers from protein concentration measurements. Toxicol In Vitro. 20:1582–1586. DOI: 10.1016/j.tiv.2006.06.003. PMID: 16930941.
Article28. Peng WC, Kraaier LJ, Kluiver TA. 2021; Hepatocyte organoids and cell transplantation: what the future holds. Exp Mol Med. 53:1512–1528. DOI: 10.1038/s12276-021-00579-x. PMID: 34663941. PMCID: PMC8568948.
Article29. Kim DS, Ryu JW, Son MY, et al. 2017; A liver-specific gene expression panel predicts the differentiation status of in vitro hepatocyte models. Hepatology. 66:1662–1674. DOI: 10.1002/hep.29324. PMID: 28640507. PMCID: PMC5698781.
Article30. Lee MO, Lee SG, Jung CR, et al. 2021; Development of a quantitative prediction algorithm for target organ-specific similarity of human pluripotent stem cell-derived organoids and cells. Nat Commun. 12:4492. DOI: 10.1038/s41467-021-24746-w. PMID: 34301945. PMCID: PMC8302568.
Article31. Duran I, Pombo J, Sun B, et al. 2024; Detection of senescence using machine learning algorithms based on nuclear features. Nat Commun. 15:1041. DOI: 10.1038/s41467-024-45421-w. PMID: 38310113. PMCID: PMC10838307.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Essential Guidelines for Manufacturing and Application of Organoids
- Guidelines for Manufacturing and Application of Organoids: Heart
- In Vitro Generation of Luminal Vasculature in Liver Organoids: From Basic Vascular Biology to Vascularized Hepatic Organoids
- Establishment and Advancement of Pancreatic Organoids
- Region Specific Brain Organoids to Study Neurodevelopmental Disorders