Int J Stem Cells.
2023 Feb;16(1):1-15. 10.15283/ijsc22154.
In Vitro Generation of Luminal Vasculature in Liver Organoids: From Basic Vascular Biology to Vascularized Hepatic Organoids
- Affiliations
-
- 1Laboratory of Stem Cells and Tissue Regeneration, Department of Biotechnology, College of Life Sciences and Biotechnology, Korea University, Seoul, Korea
- KMID: 2539616
- DOI: http://doi.org/10.15283/ijsc22154
Abstract
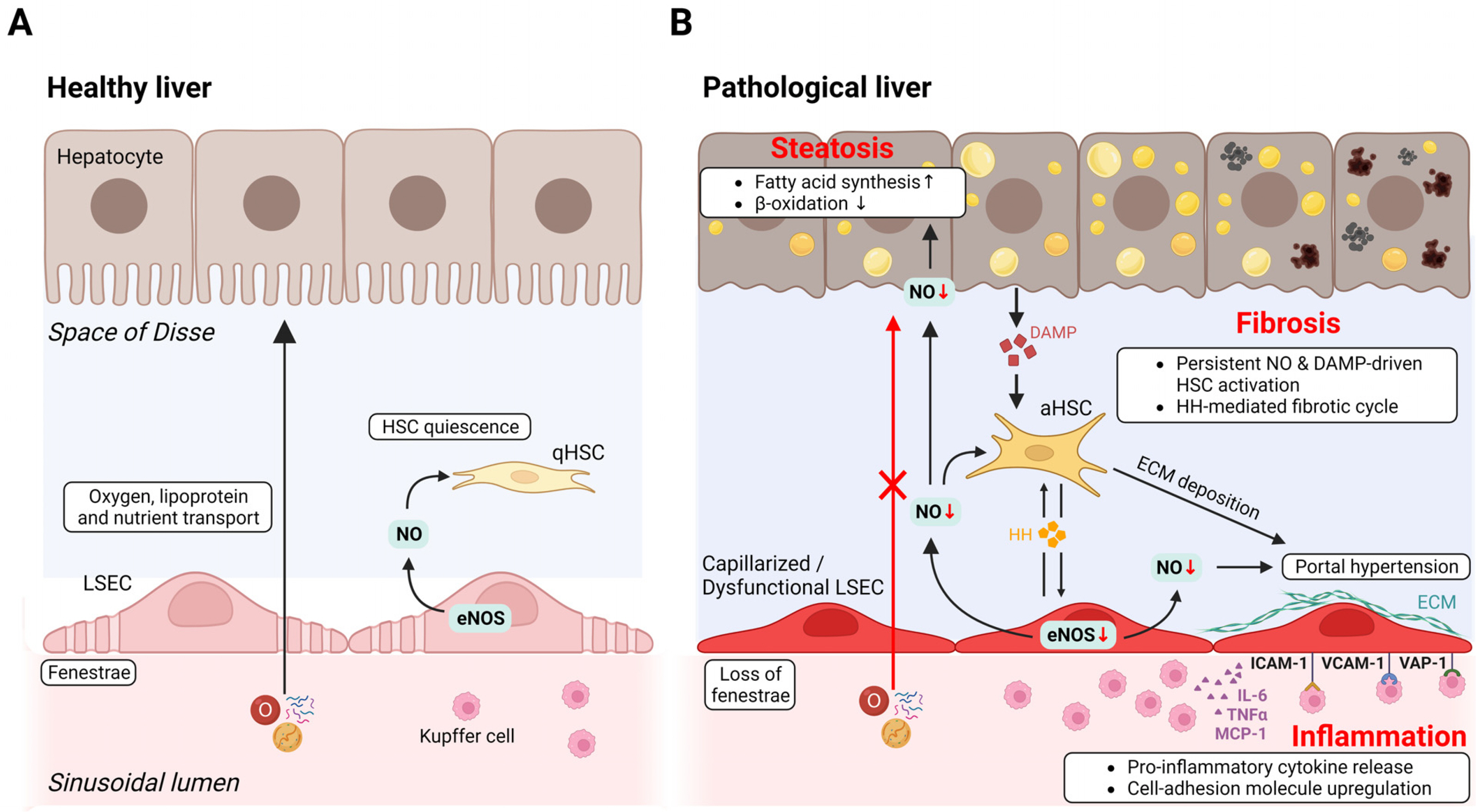
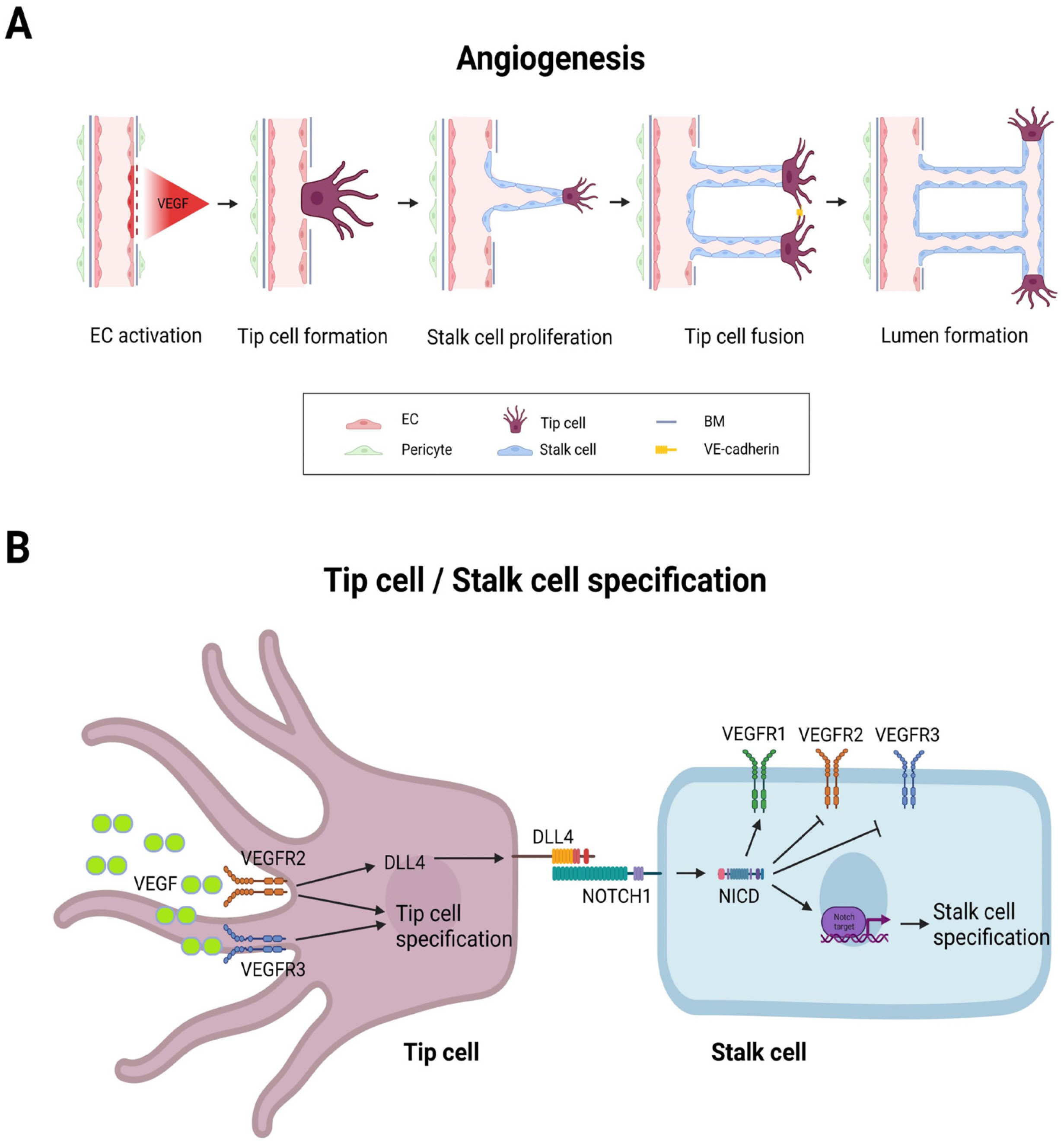
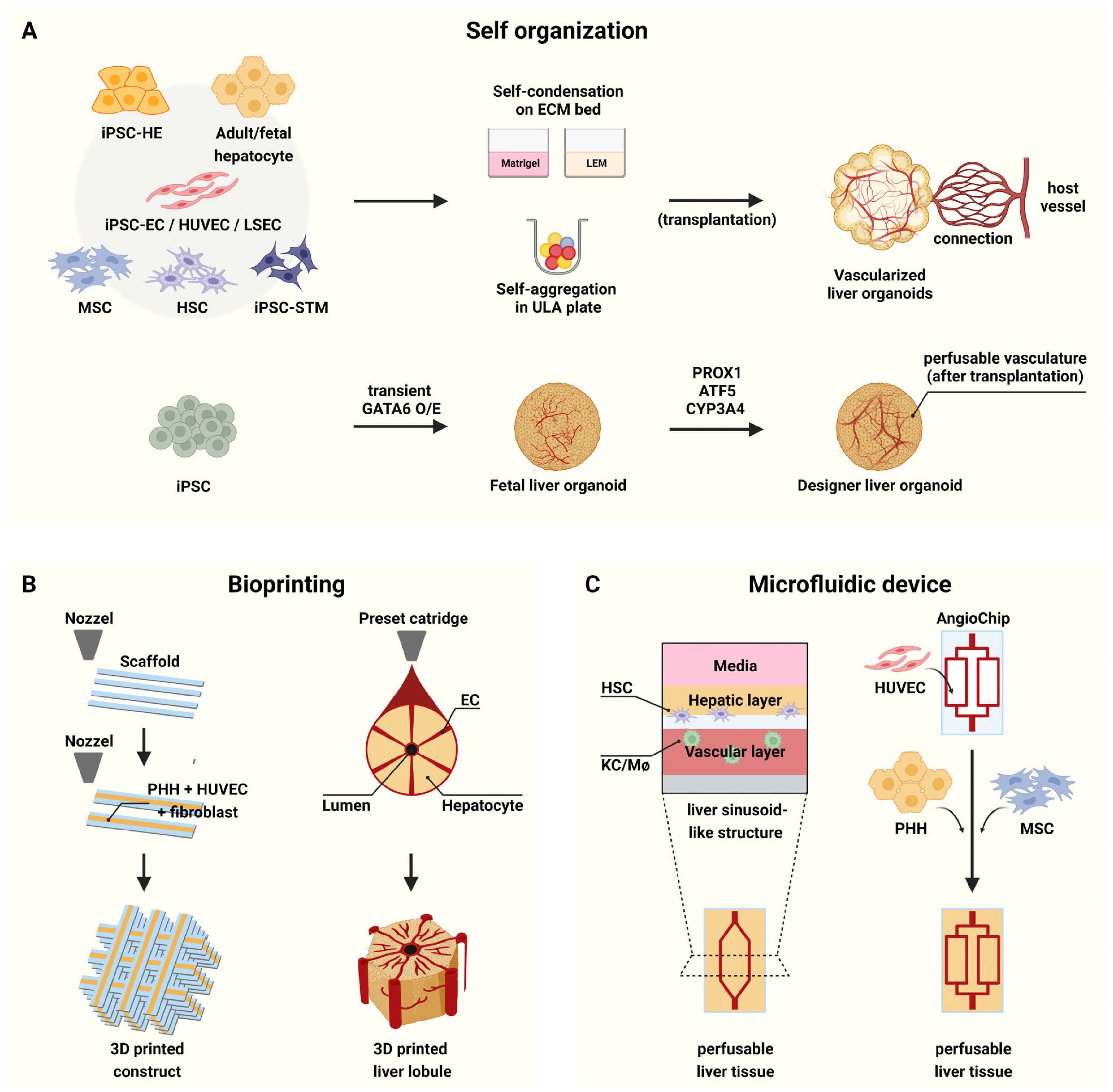
- Liver organoids have gained much attention in recent years for their potential applications to liver disease modeling and pharmacologic drug screening. Liver organoids produced In Vitro reflect some aspects of the in vivo physiological and pathological conditions of the liver. However, the generation of liver organoids with perfusable luminal vasculature remains a major challenge, hindering precise and effective modeling of liver diseases. Furthermore, vascularization is required for large organoids or assembloids to closely mimic the complexity of tissue architecture without cell death in the core region. A few studies have successfully generated liver organoids with endothelial cell networks, but most of these vascular networks produced luminal structures after being transplanted into tissues of host animals. Therefore, formation of luminal vasculature is an unmet need to overcome the limitation of liver organoids as an In Vitro model investigating different acute and chronic liver diseases. Here, we provide an overview of the unique features of hepatic vasculature under pathophysiological conditions and summarize the biochemical and biophysical cues that drive vasculogenesis and angiogenesis In Vitro. We also highlight recent progress in generating vascularized liver organoids In Vitro and discuss potential strategies that may enable the generation of perfusable luminal vasculature in liver organoids.
Keyword
Figure
Cited by 1 articles
-
Guidelines for Manufacturing and Application of Organoids: Liver
Hye-Ran Moon, Seon Ju Mun, Tae Hun Kim, Hyemin Kim, Dukjin Kang, Suran Kim, Ji Hyun Shin, Dongho Choi, Sun-Ju Ahn, Myung Jin Son
Int J Stem Cells. 2024;17(2):120-129. doi: 10.15283/ijsc24044.
Reference
-
References
1. Ho H, Zhang E. 2020; Virtual lobule models are the key for multiscale biomechanical and pharmacological modeling for the liver. Front Physiol. 11:1061. DOI: 10.3389/fphys.2020.01061. PMID: 32982791. PMCID: PMC7492636. PMID: d22d5e8caf054c85825a66c536996d6f.2. Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. 2001; Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 294:559–563. DOI: 10.1126/science.1063889. PMID: 11577199.3. Si-Tayeb K, Lemaigre FP, Duncan SA. 2010; Organogenesis and development of the liver. Dev Cell. 18:175–189. DOI: 10.1016/j.devcel.2010.01.011. PMID: 20159590.4. Iwakiri Y, Shah V, Rockey DC. 2014; Vascular pathobiology in chronic liver disease and cirrhosis - current status and future directions. J Hepatol. 61:912–924. DOI: 10.1016/j.jhep.2014.05.047. PMID: 24911462. PMCID: PMC4346093.5. Elaut G, Henkens T, Papeleu P, Snykers S, Vinken M, Vanhaecke T, Rogiers V. 2006; Molecular mechanisms underlying the dedifferentiation process of isolated hepatocytes and their cultures. Curr Drug Metab. 7:629–660. DOI: 10.2174/138920006778017759. PMID: 16918317.6. Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, Sato T, Hamer K, Sasaki N, Finegold MJ, Haft A, Vries RG, Grompe M, Clevers H. 2013; In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 494:247–250. DOI: 10.1038/nature11826. PMID: 23354049. PMCID: PMC3634804.7. Huch M, Gehart H, van Boxtel R, Hamer K, Blokzijl F, Verstegen MM, Ellis E, van Wenum M, Fuchs SA, de Ligt J, van de Wetering M, Sasaki N, Boers SJ, Kemperman H, de Jonge J, Ijzermans JN, Nieuwenhuis EE, Hoekstra R, Strom S, Vries RR, van der Laan LJ, Cuppen E, Clevers H. 2015; Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 160:299–312. DOI: 10.1016/j.cell.2014.11.050. PMID: 25533785. PMCID: PMC4313365.8. Hu H, Gehart H, Artegiani B, LÖpez-Iglesias C, Dekkers F, Basak O, van Es J, Chuva de Sousa Lopes SM, Begthel H, Korving J, van den Born M, Zou C, Quirk C, Chiriboga L, Rice CM, Ma S, Rios A, Peters PJ, de Jong YP, Clevers H. 2018; Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell. 175:1591–1606.e19. DOI: 10.1016/j.cell.2018.11.013. PMID: 30500538.9. Shinozawa T, Kimura M, Cai Y, Saiki N, Yoneyama Y, Ouchi R, Koike H, Maezawa M, Zhang RR, Dunn A, Ferguson A, Togo S, Lewis K, Thompson WL, Asai A, Takebe T. 2021; High-fidelity drug-induced liver injury screen using human pluripotent stem cell-derived organoids. Gast-roenterology. 160:831–846.e10. DOI: 10.1053/j.gastro.2020.10.002. PMID: 33039464. PMCID: PMC7878295.10. Kim H, Im I, Jeon JS, Kang EH, Lee HA, Jo S, Kim JW, Woo DH, Choi YJ, Kim HJ, Han JS, Lee BS, Kim JH, Kim SK, Park HJ. 2022; Development of human pluripotent stem cell-derived hepatic organoids as an alternative model for drug safety assessment. Biomaterials. 286:121575. DOI: 10.1016/j.biomaterials.2022.121575. PMID: 35598335.11. Mun SJ, Ryu JS, Lee MO, Son YS, Oh SJ, Cho HS, Son MY, Kim DS, Kim SJ, Yoo HJ, Lee HJ, Kim J, Jung CR, Chung KS, Son MJ. 2019; Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J Hepatol. 71:970–985. DOI: 10.1016/j.jhep.2019.06.030. PMID: 31299272.12. Collardeau-Frachon S, Scoazec JY. 2008; Vascular development and differentiation during human liver organogenesis. Anat Rec (Hoboken). 291:614–627. DOI: 10.1002/ar.20679. PMID: 18484606.13. Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, Aoyama S, Adachi Y, Taniguchi H. 2013; Vascularized and functional human liver from an iPSC-derived organ bud trans-plant. Nature. 499:481–484. DOI: 10.1038/nature12271. PMID: 23823721.14. Camp JG, Sekine K, Gerber T, Loeffler-Wirth H, Binder H, Gac M, Kanton S, Kageyama J, Damm G, Seehofer D, Belicova L, Bickle M, Barsacchi R, Okuda R, Yoshizawa E, Kimura M, Ayabe H, Taniguchi H, Takebe T, Treutlein B. 2017; Multilineage communication regulates human liver bud development from pluripotency. Nature. 546:533–538. DOI: 10.1038/nature22796. PMID: 28614297.15. Jain RK, Au P, Tam J, Duda DG, Fukumura D. 2005; Engi-neering vascularized tissue. Nat Biotechnol. 23:821–823. DOI: 10.1038/nbt0705-821. PMID: 16003365.16. Takebe T, Sekine K, Kimura M, Yoshizawa E, Ayano S, Koido M, Funayama S, Nakanishi N, Hisai T, Kobayashi T, Kasai T, Kitada R, Mori A, Ayabe H, Ejiri Y, Amimoto N, Yamazaki Y, Ogawa S, Ishikawa M, Kiyota Y, Sato Y, Nozawa K, Okamoto S, Ueno Y, Taniguchi H. 2017; Massive and reproducible production of liver buds entirely from human pluripotent stem cells. Cell Rep. 21:2661–2670. DOI: 10.1016/j.celrep.2017.11.005. PMID: 29212014.17. Velazquez JJ, LeGraw R, Moghadam F, Tan Y, Kilbourne J, Maggiore JC, Hislop J, Liu S, Cats D, Chuva de Sousa Lopes SM, Plaisier C, Cahan P, Kiani S, Ebrahimkhani MR. 2021; Gene regulatory network analysis and engineering directs development and vascularization of multilineage human liver organoids. Cell Syst. 12:41–55.e11. DOI: 10.1016/j.cels.2020.11.002. PMID: 33290741. PMCID: PMC8164844.18. Rafii S, Butler JM, Ding BS. 2016; Angiocrine functions of organ-specific endothelial cells. Nature. 529:316–325. DOI: 10.1038/nature17040. PMID: 26791722. PMCID: PMC4878406.19. Trefts E, Gannon M, Wasserman DH. 2017; The liver. Curr Biol. 27:R1147–R1151. DOI: 10.1016/j.cub.2017.09.019. PMID: 29112863. PMCID: PMC5897118.20. DeSesso JM. 2017; Vascular ontogeny within selected thoracoab-dominal organs and the limbs. Reprod Toxicol. 70:3–20. DOI: 10.1016/j.reprotox.2016.10.007. PMID: 27810254.21. Zhang X, Tang L, Yi Q. 2021; Engineering the vasculature of stem-cell-derived liver organoids. Biomolecules. 11:966. DOI: 10.3390/biom11070966. PMID: 34208902. PMCID: PMC8301828. PMID: e4a3667bd3fc4fcbb9204a3fe6a20c91.22. Poisson J, Lemoinne S, Boulanger C, Durand F, Moreau R, Valla D, Rautou PE. 2017; Liver sinusoidal endothelial cells: physiology and role in liver diseases. J Hepatol. 66:212–227. DOI: 10.1016/j.jhep.2016.07.009. PMID: 27423426.23. Ober EA, Lemaigre FP. 2018; Development of the liver: insights into organ and tissue morphogenesis. J Hepatol. 68:1049–1062. DOI: 10.1016/j.jhep.2018.01.005. PMID: 29339113.24. DeLeve LD, Wang X, Hu L, McCuskey MK, McCuskey RS. 2004; Rat liver sinusoidal endothelial cell phenotype is maintained by paracrine and autocrine regulation. Am J Physiol Gastrointest Liver Physiol. 287:G757–G763. DOI: 10.1152/ajpgi.00017.2004. PMID: 15191879.25. Yoshida M, Nishikawa Y, Omori Y, Yoshioka T, Tokairin T, McCourt P, Enomoto K. 2007; Involvement of signaling of VEGF and TGF-beta in differentiation of sinusoidal endothelial cells during culture of fetal rat liver cells. Cell Tissue Res. 329:273–282. DOI: 10.1007/s00441-007-0387-5. PMID: 17450384.26. Klein D, Demory A, Peyre F, Kroll J, Augustin HG, Helfrich W, Kzhyshkowska J, Schledzewski K, Arnold B, Goerdt S. 2008; Wnt2 acts as a cell type-specific, autocrine gro-wth factor in rat hepatic sinusoidal endothelial cells cross-stimulating the VEGF pathway. Hepatology. 47:1018–1031. DOI: 10.1002/hep.22084. PMID: 18302287.27. Géraud C, Koch PS, Zierow J, Klapproth K, Busch K, Olsavszky V, Leibing T, Demory A, Ulbrich F, Diett M, Singh S, Sticht C, Breitkopf-Heinlein K, Richter K, Karppinen SM, Pihlajaniemi T, Arnold B, Rodewald HR, Augustin HG, Schledzewski K, Goerdt S. 2017; GATA4-dependent organ-specific endothelial differentiation controls liver development and embryonic hematopoiesis. J Clin Invest. 127:1099–1114. DOI: 10.1172/JCI90086. PMID: 28218627. PMCID: PMC5330741.28. DeLeve LD, Maretti-Mira AC. 2017; Liver sinusoidal endothelial cell: an update. Semin Liver Dis. 37:377–387. DOI: 10.1055/s-0037-1617455. PMID: 29272898. PMCID: PMC6005648.29. Hammoutene A, Rautou PE. 2019; Role of liver sinusoidal endothelial cells in non-alcoholic fatty liver disease. J Hepatol. 70:1278–1291. DOI: 10.1016/j.jhep.2019.02.012. PMID: 30797053.30. Gracia-Sancho J, Caparrós E, Fernández-Iglesias A, Francés R. 2021; Role of liver sinusoidal endothelial cells in liver diseases. Nat Rev Gastroenterol Hepatol. 18:411–431. DOI: 10.1038/s41575-020-00411-3. PMID: 33589830.31. Gracia-Sancho J, Laviña B, Rodríguez-Vilarrupla A, García-Calderó H, Bosch J, García-Pagán JC. 2007; Enhanced vasoconstrictor prostanoid production by sinusoidal endothelial cells increases portal perfusion pressure in cirrhotic rat livers. J Hepatol. 47:220–227. DOI: 10.1016/j.jhep.2007.03.014. PMID: 17459512.32. Deleve LD, Wang X, Guo Y. 2008; Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology. 48:920–930. DOI: 10.1002/hep.22351. PMID: 18613151. PMCID: PMC2695448.33. Xie G, Choi SS, Syn WK, Michelotti GA, Swiderska M, Karaca G, Chan IS, Chen Y, Diehl AM. 2013; Hedgehog signalling regulates liver sinusoidal endothelial cell capillari-sation. Gut. 62:299–309. DOI: 10.1136/gutjnl-2011-301494. PMID: 22362915. PMCID: PMC3595101.34. Ding BS, Cao Z, Lis R, Nolan DJ, Guo P, Simons M, Penfold ME, Shido K, Rabbany SY, Rafii S. 2014; Divergent angiocrine signals from vascular niche balance liver rege-neration and fibrosis. Nature. 505:97–102. DOI: 10.1038/nature12681. PMID: 24256728. PMCID: PMC4142699.35. Coulon S, Heindryckx F, Geerts A, Van Steenkiste C, Colle I, Van Vlierberghe H. 2011; Angiogenesis in chronic liver disease and its complications. Liver Int. 31:146–162. DOI: 10.1111/j.1478-3231.2010.02369.x. PMID: 21073649.36. Patan S. 2004; Vasculogenesis and angiogenesis. Cancer Treat Res. 117:3–32. DOI: 10.1007/978-1-4419-8871-3_1. PMID: 15015550.37. Potente M, Mäkinen T. 2017; Vascular heterogeneity and specialization in development and disease. Nat Rev Mol Cell Biol. 18:477–494. DOI: 10.1038/nrm.2017.36. PMID: 28537573.38. Arroyo AG, Iruela-Arispe ML. 2010; Extracellular matrix, inflammation, and the angiogenic response. Cardiovasc Res. 86:226–235. DOI: 10.1093/cvr/cvq049. PMID: 20154066. PMCID: PMC2856193.39. Potente M, Gerhardt H, Carmeliet P. 2011; Basic and therapeutic aspects of angiogenesis. Cell. 146:873–887. DOI: 10.1016/j.cell.2011.08.039. PMID: 21925313.40. Naito H, Iba T, Takakura N. 2020; Mechanisms of new blood-vessel formation and proliferative heterogeneity of endothelial cells. Int Immunol. 32:295–305. DOI: 10.1093/intimm/dxaa008. PMID: 31996897.41. Choi HJ, Zhang H, Park H, Choi KS, Lee HW, Agrawal V, Kim YM, Kwon YG. 2015; Yes-associated protein regulates endothelial cell contact-mediated expression of angiopoietin-2. Nat Commun. 6:6943. DOI: 10.1038/ncomms7943. PMID: 25962877.42. Ong YT, Andrade J, Armbruster M, Shi C, Castro M, Costa ASH, Sugino T, Eelen G, Zimmermann B, Wilhelm K, Lim J, Watanabe S, Guenther S, Schneider A, Zanconato F, Kaulich M, Pan D, Braun T, Gerhardt H, Efeyan A, Carmeliet P, Piccolo S, Grosso AR, Potente M. 2022; A YAP/TAZ-TEAD signalling module links endothelial nutrient acquisition to angiogenic growth. Nat Metab. 4:672–682. DOI: 10.1038/s42255-022-00584-y. PMID: 35726026. PMCID: PMC9236904.43. Wright GL, Maroulakou IG, Eldridge J, Liby TL, Sridharan V, Tsichlis PN, Muise-Helmericks RC. 2008; VEGF stimulation of mitochondrial biogenesis: requirement of AKT3 kinase. FASEB J. 22:3264–3275. DOI: 10.1096/fj.08-106468. PMID: 18524868. PMCID: PMC2518259.44. Guo D, Wang Q, Li C, Wang Y, Chen X. 2017; VEGF stimulated the angiogenesis by promoting the mitochondrial functions. Oncotarget. 8:77020–77027. DOI: 10.18632/oncotarget.20331. PMID: 29100366. PMCID: PMC5652760.45. Lamalice L, Le Boeuf F, Huot J. 2007; Endothelial cell migration during angiogenesis. Circ Res. 100:782–794. DOI: 10.1161/01.RES.0000259593.07661.1e. PMID: 17395884.46. Serbo JV, Gerecht S. 2013; Vascular tissue engineering: biodegradable scaffold platforms to promote angiogenesis. Stem Cell Res Ther. 4:8. DOI: 10.1186/scrt156. PMID: 23347554. PMCID: PMC3706776.47. Saharinen P, Eklund L, Miettinen J, Wirkkala R, Anisimov A, Winderlich M, Nottebaum A, Vestweber D, Deutsch U, Koh GY, Olsen BR, Alitalo K. 2008; Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat Cell Biol. 10:527–537. DOI: 10.1038/ncb1715. PMID: 18425119.48. Sulpice E, Ding S, Muscatelli-Groux B, Bergé M, Han ZC, Plouet J, Tobelem G, Merkulova-Rainon T. 2009; Cross-talk between the VEGF-A and HGF signalling pathways in endothelial cells. Biol Cell. 101:525–539. DOI: 10.1042/BC20080221. PMID: 19281453.49. Kaga T, Kawano H, Sakaguchi M, Nakazawa T, Taniyama Y, Morishita R. 2012; Hepatocyte growth factor stimulated angiogenesis without inflammation: differential actions between hepatocyte growth factor, vascular endothelial growth factor and basic fibroblast growth factor. Vascul Pharmacol. 57:3–9. DOI: 10.1016/j.vph.2012.02.002. PMID: 22361334.50. Oladipupo S, Hu S, Kovalski J, Yao J, Santeford A, Sohn RE, Shohet R, Maslov K, Wang LV, Arbeit JM. 2011; VEGF is essential for hypoxia-inducible factor-mediated neovascu-larization but dispensable for endothelial sprouting. Proc Natl Acad Sci U S A. 108:13264–13269. DOI: 10.1073/pnas.1101321108. PMID: 21784979. PMCID: PMC3156154.51. Brindle NP, Saharinen P, Alitalo K. 2006; Signaling and functions of angiopoietin-1 in vascular protection. Circ Res. 98:1014–1023. DOI: 10.1161/01.RES.0000218275.54089.12. PMID: 16645151. PMCID: PMC2270395.52. Williams PA, Stilhano RS, To VP, Tran L, Wong K, Silva EA. 2015; Hypoxia augments outgrowth endothelial cell (OEC) sprouting and directed migration in response to sphingosine-1-phosphate (S1P). PLoS One. 10:e0123437. DOI: 10.1371/journal.pone.0123437. PMID: 25875493. PMCID: PMC4398361.53. Qin D, Trenkwalder T, Lee S, Chillo O, Deindl E, Kupatt C, Hinkel R. 2013; Early vessel destabilization mediated by Angiopoietin-2 and subsequent vessel maturation via Angi-opoietin-1 induce functional neovasculature after ischemia. PLoS One. 8:e61831. DOI: 10.1371/journal.pone.0061831. PMID: 23613948. PMCID: PMC3628915. PMID: 0d4ab9496c834c99b15ebe93bd774f45.54. Takuwa Y, Du W, Qi X, Okamoto Y, Takuwa N, Yoshioka K. 2010; Roles of sphingosine-1-phosphate signaling in angio-genesis. World J Biol Chem. 1:298–306. DOI: 10.4331/wjbc.v1.i10.298. PMID: 21537463. PMCID: PMC3083935.55. Crosby CO, Zoldan J. 2019; Mimicking the physical cues of the ECM in angiogenic biomaterials. Regen Biomater. 6:61–73. DOI: 10.1093/rb/rbz003. PMID: 30967961. PMCID: PMC6447000.56. Edgar LT, Underwood CJ, Guilkey JE, Hoying JB, Weiss JA. 2014; Extracellular matrix density regulates the rate of neovessel growth and branching in sprouting angiogenesis. PLoS One. 9:e85178. DOI: 10.1371/journal.pone.0085178. PMID: 24465500. PMCID: PMC3898992.57. Mason BN, Starchenko A, Williams RM, Bonassar LJ, Reinhart-King CA. 2013; Tuning three-dimensional collagen matrix stiffness independently of collagen concentration modulates endothelial cell behavior. Acta Biomater. 9:4635–4644. DOI: 10.1016/j.actbio.2012.08.007. PMID: 22902816. PMCID: PMC3508162.58. Bao M, Chen Y, Liu JT, Bao H, Wang WB, Qi YX, Lv F. 2022; Extracellular matrix stiffness controls VEGF165 secretion and neuroblastoma angiogenesis via the YAP/RUNX2/SRSF1 axis. Angiogenesis. 25:71–86. DOI: 10.1007/s10456-021-09804-7. PMID: 34170441.59. Guo Y, Mei F, Huang Y, Ma S, Wei Y, Zhang X, Xu M, He Y, Heng BC, Chen L, Deng X. 2021; Matrix stiffness modulates tip cell formation through the p-PXN-Rac1-YAP signaling axis. Bioact Mater. 7:364–376. DOI: 10.1016/j.bioactmat.2021.05.033. PMID: 34466738. PMCID: PMC8379356.60. Paszkowiak JJ, Dardik A. 2003; Arterial wall shear stress: observations from the bench to the bedside. Vasc Endo-vascular Surg. 37:47–57. DOI: 10.1177/153857440303700107. PMID: 12577139.61. Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, Pannekoek H, Horrevoets AJ. 2002; Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Krüppel-like factor (KLF2). Blood. 100:1689–1698. DOI: 10.1182/blood-2002-01-0046. PMID: 12176889.62. Yuan P, Hu Q, He X, Long Y, Song X, Wu F, He Y, Zhou X. 2020; Laminar flow inhibits the Hippo/YAP pathway via autophagy and SIRT1-mediated deacetylation against athero-sclerosis. Cell Death Dis. 11:141. DOI: 10.1038/s41419-020-2343-1. PMID: 32081881. PMCID: PMC7035362.63. Takebe T, Koike N, Sekine K, Fujiwara R, Amiya T, Zheng YW, Taniguchi H. 2014; Engineering of human hepatic tissue with functional vascular networks. Organogenesis. 10:260–267. DOI: 10.4161/org.27590. PMID: 24451152. PMCID: PMC4154961.64. Ramachandran SD, Schirmer K, Münst B, Heinz S, Ghafoory S, Wölfl S, Simon-Keller K, Marx A, Øie CI, Ebert MP, Walles H, Braspenning J, Breitkopf-Heinlein K. 2015; In vitro generation of functional liver organoid-like structures using adult human cells. PLoS One. 10:e0139345. DOI: 10.1371/journal.pone.0139345. PMID: 26488607. PMCID: PMC4619350.65. Li J, Xing F, Chen F, He L, So KF, Liu Y, Xiao J. 2019; Functional 3D human liver bud assembled from MSC-derived multiple liver cell lineages. Cell Transplant. 28:510–521. Erratum in: Cell Transplant 2022;31: 9636897221126859. DOI: 10.1177/0963689718780332. PMID: 29895168. PMCID: PMC7103600. PMID: fb210640b4f34726b211f3e347a4474a.66. Takebe T, Enomura M, Yoshizawa E, Kimura M, Koike H, Ueno Y, Matsuzaki T, Yamazaki T, Toyohara T, Osafune K, Nakauchi H, Yoshikawa HY, Taniguchi H. 2015; Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation. Cell Stem Cell. 16:556–565. DOI: 10.1016/j.stem.2015.03.004. PMID: 25891906.67. Asai A, Aihara E, Watson C, Mourya R, Mizuochi T, Shivakumar P, Phelan K, Mayhew C, Helmrath M, Takebe T, Wells J, Bezerra JA. 2017; Paracrine signals regulate human liver organoid maturation from induced pluripotent stem cells. Development. 144:1056–1064. DOI: 10.1242/dev.142794. PMID: 28275009. PMCID: PMC5358109.68. Ayabe H, Anada T, Kamoya T, Sato T, Kimura M, Yoshizawa E, Kikuchi S, Ueno Y, Sekine K, Camp JG, Treutlein B, Ferguson A, Suzuki O, Takebe T, Taniguchi H. 2018; Optimal hypoxia regulates human iPSC-derived liver bud differentiation through intercellular TGFB signaling. Stem Cell Reports. 11:306–316. DOI: 10.1016/j.stemcr.2018.06.015. PMID: 30033085. PMCID: PMC6092760.69. Goulart E, de Caires-Junior LC, Telles-Silva KA, Araujo BHS, Kobayashi GS, Musso CM, Assoni AF, Oliveira D, Caldini E, Gerstenhaber JA, Raia S, Lelkes PI, Zatz M. 2019; Adult and iPS-derived non-parenchymal cells regulate liver organoid development through differential modulation of Wnt and TGF-β. Stem Cell Res Ther. 10:258. DOI: 10.1186/s13287-019-1367-x. PMID: 31416480. PMCID: PMC6694663. PMID: 4ba337cc5eb2432daa9b792de03a1394.70. Jin Y, Kim J, Lee JS, Min S, Kim S, Ahn DH, Kim YG, Cho SW. 2018; Vascularized liver organoids generated using induced hepatic tissue and dynamic liver-specific microenvironment as a drug testing platform. Adv Funct Mater. 28:1801954. DOI: 10.1002/adfm.201801954.71. Peng WC, Logan CY, Fish M, Anbarchian T, Aguisanda F, Álvarez-Varela A, Wu P, Jin Y, Zhu J, Li B, Grompe M, Wang B, Nusse R. 2018; Inflammatory cytokine TNFα promotes the long-term expansion of primary hepatocytes in 3D culture. Cell. 175:1607–1619.e15. DOI: 10.1016/j.cell.2018.11.012. PMID: 30500539. PMCID: PMC6497386.72. Ardalani H, Sengupta S, Harms V, Vickerman V, Thomson JA, Murphy WL. 2019; 3-D culture and endothelial cells improve maturity of human pluripotent stem cell-derived hepato-cytes. Acta Biomater. 95:371–381. DOI: 10.1016/j.actbio.2019.07.047. PMID: 31362140.73. Yap KK, Gerrand YW, Dingle AM, Yeoh GC, Morrison WA, Mitchell GM. 2020; Liver sinusoidal endothelial cells promote the differentiation and survival of mouse vascularised hepatobiliary organoids. Biomaterials. 251:120091. DOI: 10.1016/j.biomaterials.2020.120091. PMID: 32408048.74. Son JS, Park CY, Lee G, Park JY, Kim HJ, Kim G, Chi KY, Woo DH, Han C, Kim SK, Park HJ, Kim DW, Kim JH. 2022; Therapeutic correction of hemophilia A using 2D endothelial cells and multicellular 3D organoids derived from CRISPR/Cas9-engineered patient iPSCs. Biomaterials. 283:121429. DOI: 10.1016/j.biomaterials.2022.121429. PMID: 35217482.75. Sasaki K, Akagi T, Asaoka T, Eguchi H, Fukuda Y, Iwagami Y, Yamada D, Noda T, Wada H, Gotoh K, Kawamoto K, Doki Y, Mori M, Akashi M. 2017; Construction of three-dimensional vascularized functional human liver tissue using a layer-by-layer cell coating technique. Bioma-terials. 133:263–274. DOI: 10.1016/j.biomaterials.2017.02.034. PMID: 28448819.76. Guye P, Ebrahimkhani MR, Kipniss N, Velazquez JJ, Schoenfeld E, Kiani S, Griffith LG, Weiss R. 2016; Genetically engineering self-organization of human pluripotent stem cells into a liver bud-like tissue using Gata6. Nat Commun. 7:10243. DOI: 10.1038/ncomms10243. PMID: 26732624. PMCID: PMC4729822. PMID: 9b8be1df7dff49edbe97c27e4110a2e7.77. Lee JW, Choi YJ, Yong WJ, Pati F, Shim JH, Kang KS, Kang IH, Park J, Cho DW. 2016; Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering. Biofabrication. 8:015007. DOI: 10.1088/1758-5090/8/1/015007. PMID: 26756962.78. Kang D, Hong G, An S, Jang I, Yun WS, Shim JH, Jin S. 2020; Bioprinting of multiscaled hepatic lobules within a highly vascularized construct. Small. 16:e1905505. DOI: 10.1002/smll.201905505. PMID: 32078240.79. Yanagi Y, Nakayama K, Taguchi T, Enosawa S, Tamura T, Yoshimaru K, Matsuura T, Hayashida M, Kohashi K, Oda Y, Yamaza T, Kobayashi E. 2017; In vivo and ex vivo methods of growing a liver bud through tissue connection. Sci Rep. 7:14085. DOI: 10.1038/s41598-017-14542-2. PMID: 29074999. PMCID: PMC5658340. PMID: a949c20ed63347e9bb0e8d510aa546e4.80. Rennert K, Steinborn S, Gröger M, Ungerböck B, Jank AM, Ehgartner J, Nietzsche S, Dinger J, Kiehntopf M, Funke H, Peters FT, Lupp A, Gärtner C, Mayr T, Bauer M, Huber O, Mosig AS. 2015; A microfluidically perfused three dimensional human liver model. Biomaterials. 71:119–131. DOI: 10.1016/j.biomaterials.2015.08.043. PMID: 26322723.81. Li X, George SM, Vernetti L, Gough AH, Taylor DL. 2018; A glass-based, continuously zonated and vascularized human liver acinus microphysiological system (vLAMPS) designed for experimental modeling of diseases and ADME/TOX. Lab Chip. 18:2614–2631. DOI: 10.1039/C8LC00418H. PMID: 30063238. PMCID: PMC6113686.82. Zhang B, Montgomery M, Chamberlain MD, Ogawa S, Korolj A, Pahnke A, Wells LA, Massé S, Kim J, Reis L, Momen A, Nunes SS, Wheeler AR, Nanthakumar K, Keller G, Sefton MV, Radisic M. 2016; Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat Mater. 15:669–678. DOI: 10.1038/nmat4570. PMID: 26950595. PMCID: PMC4879054.83. Baptista PM, Siddiqui MM, Lozier G, Rodriguez SR, Atala A, Soker S. 2011; The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 53:604–617. DOI: 10.1002/hep.24067. PMID: 21274881.84. Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, Mau D, Valerius MT, Ferrante T, Bonventre JV, Lewis JA, Morizane R. 2019; Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods. 16:255–262. DOI: 10.1038/s41592-019-0325-y. PMID: 30742039. PMCID: PMC6488032.85. Cakir B, Xiang Y, Tanaka Y, Kural MH, Parent M, Kang YJ, Chapeton K, Patterson B, Yuan Y, He CS, Raredon MSB, Dengelegi J, Kim KY, Sun P, Zhong M, Lee S, Patra P, Hyder F, Niklason LE, Lee SH, Yoon YS, Park IH. 2019; Engineering of human brain organoids with a functional vascular-like system. Nat Methods. 16:1169–1175. DOI: 10.1038/s41592-019-0586-5. PMID: 31591580. PMCID: PMC6918722.86. Sun XY, Ju XC, Li Y, Zeng PM, Wu J, Zhou YY, Shen LB, Dong J, Chen YJ, Luo ZG. 2022; Generation of vascularized brain organoids to study neurovascular interactions. Elife. 11:e76707. DOI: 10.7554/eLife.76707. PMID: 35506651. PMCID: PMC9246368. PMID: b811a554852a4f8faceda71a6345dc1e.87. Kook MG, Lee SE, Shin N, Kong D, Kim DH, Kim MS, Kang HK, Choi SW, Kang KS. 2022; Generation of cortical brain organoid with vascularization by assembling with vascular spheroid. Int J Stem Cells. 15:85–94. DOI: 10.15283/ijsc21157. PMID: 35220294. PMCID: PMC8889335.88. Heo JH, Kang D, Seo SJ, Jin Y. 2022; Engineering the extracellular matrix for organoid culture. Int J Stem Cells. 15:60–69. DOI: 10.15283/ijsc21190. PMID: 35220292. PMCID: PMC8889330.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vascularized Organoids: A More Complete Model
- Generation of Cortical Brain Organoid with Vascularization by Assembling with Vascular Spheroid
- Enhancing generation efficiency of liver organoids in a collagen scaffold using human chemically derived hepatic progenitors
- Liver Organoids: Formation Strategies and Biomedical Applications
- Establishment and Advancement of Pancreatic Organoids