Clin Endosc.
2024 May;57(3):283-292. 10.5946/ce.2023.087.
E-learning system to improve the endoscopic diagnosis of early gastric cancer
- Affiliations
-
- 1Department of Endoscopy, Fukuoka University Chikushi Hospital, Fukuoka, Japan
- 2Department of Human Pathology, Juntendo University Graduate School of Medicine, Tokyo, Japan
- 3Department of Gastrointestinal Oncology, Osaka International Cancer Institute, Osaka, Japan
- 4Department of Gastroenterology, Ishikawa Prefectural Central Hospital, Kanazawa, Japan
- 5Department of Molecular-Targeting Cancer Prevention, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Osaka, Japan
- 6Department of Pathology, Fukuoka University Chikushi Hospital, Fukuoka, Japan
- 7Department of Development, FAST Inc., Tokyo, Japan
- KMID: 2555888
- DOI: http://doi.org/10.5946/ce.2023.087
Abstract
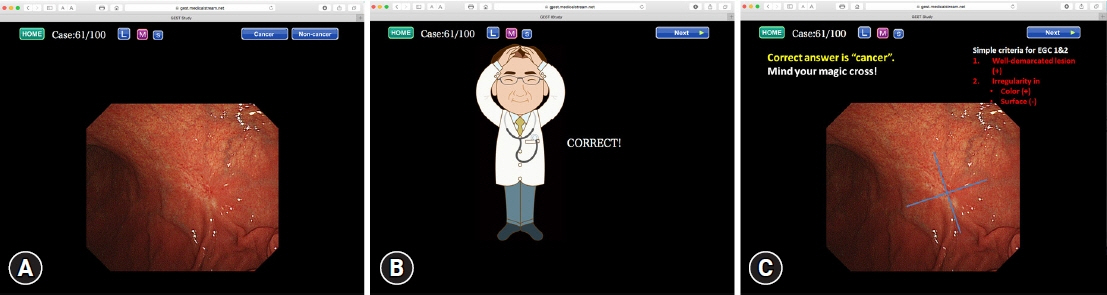
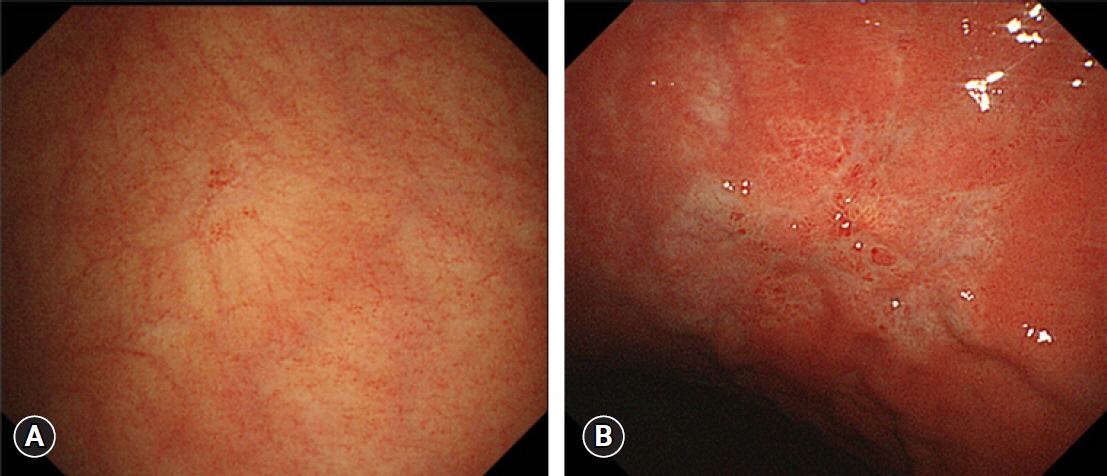
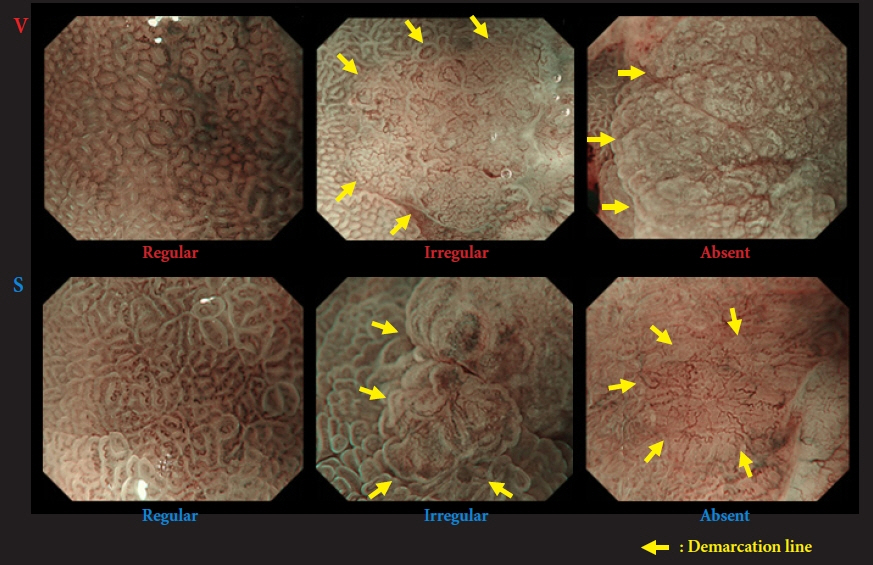
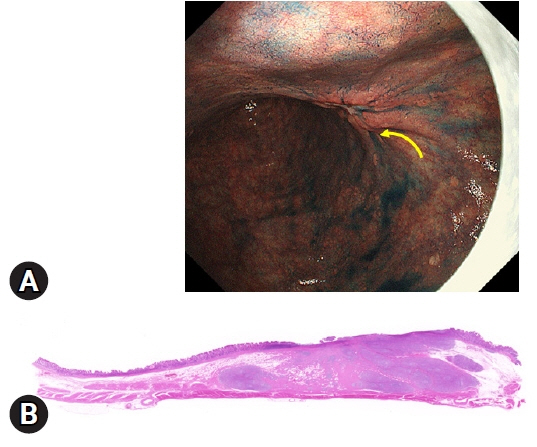
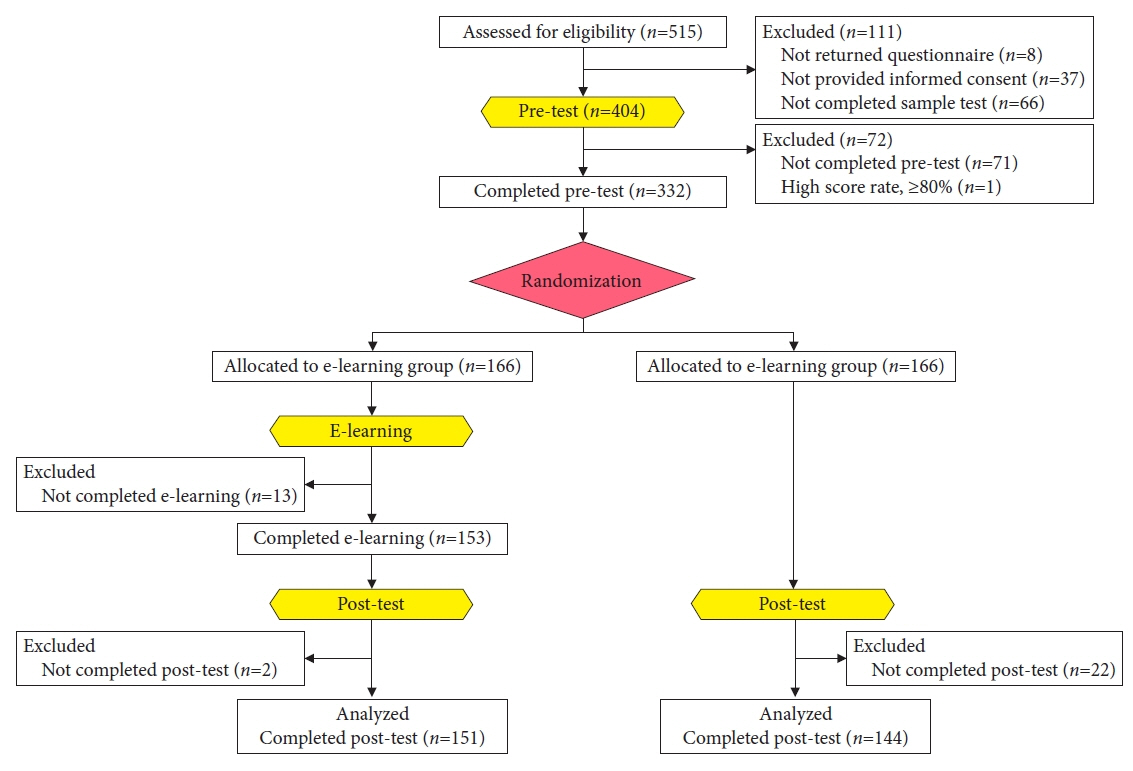
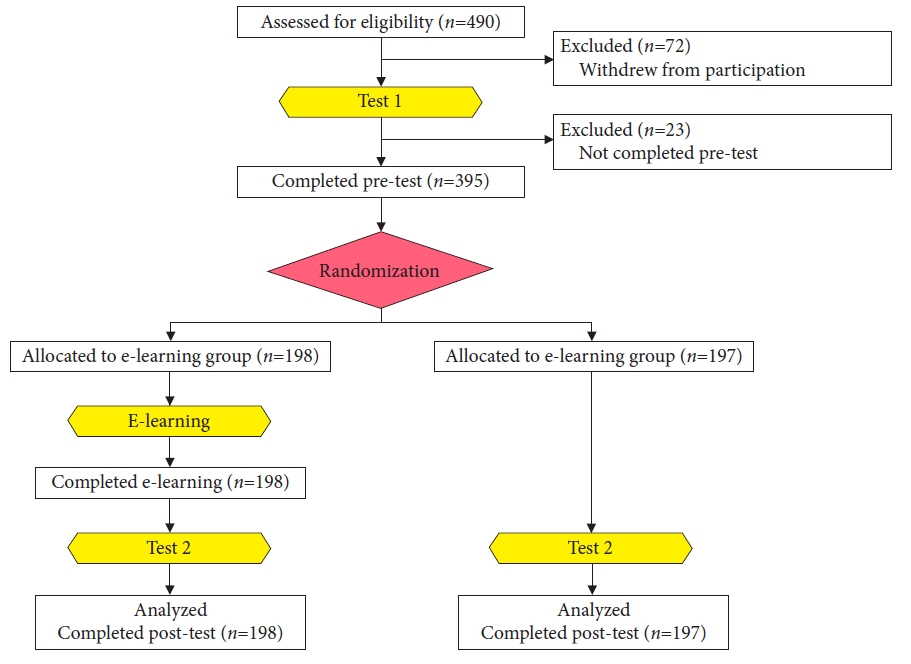
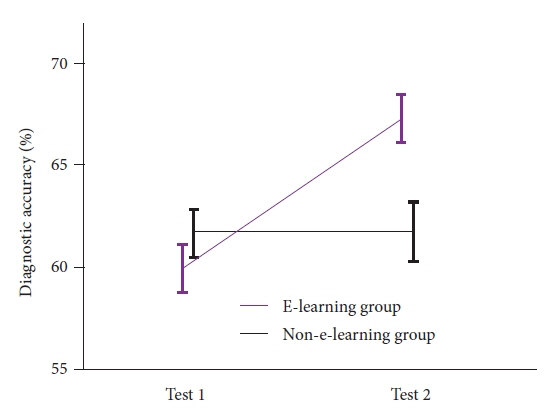
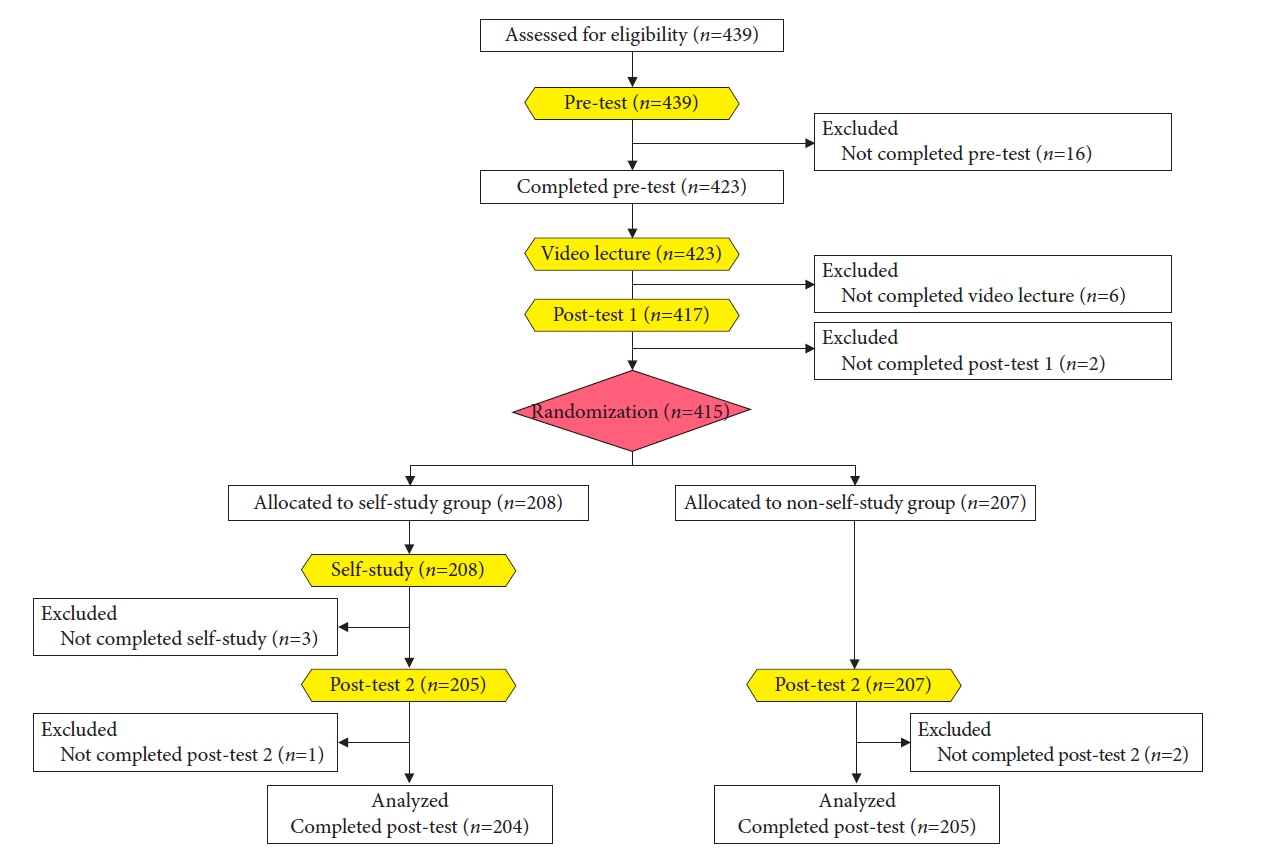
- We developed three e-learning systems for endoscopists to acquire the necessary skills to improve the diagnosis of early gastric cancer (EGC) and demonstrated their usefulness using randomized controlled trials. The subjects of the three e-learning systems were “detection”, “characterization”, and “preoperative assessment”. The contents of each e-learning system included “technique”, “knowledge”, and “obtaining experience”. All e-learning systems proved useful for endoscopists to learn how to diagnose EGC. Lecture videos describing “the technique” and “the knowledge” can be beneficial. In addition, repeating 100 self-study cases allows learners to gain “experience” and improve their diagnostic skills further. Web-based e-learning systems have more advantages than other teaching methods because the number of participants is unlimited. Histopathological diagnosis is the gold standard for the diagnosis of gastric cancer. Therefore, we developed a comprehensive diagnostic algorithm to standardize the histopathological diagnosis of gastric cancer. Once we have successfully shown that this algorithm is helpful for the accurate histopathological diagnosis of cancer, we will complete a series of e-learning systems designed to assess EGC accurately.
Keyword
Figure
Reference
-
1. Yang L, Ying X, Liu S, et al. Gastric cancer: Epidemiology, risk factors and prevention strategies. Chin J Cancer Res. 2020; 32:695–704.
Article2. Hasuike N, Ono H, Boku N, et al. A non-randomized confirmatory trial of an expanded indication for endoscopic submucosal dissection for intestinal-type gastric cancer (cT1a): the Japan Clinical Oncology Group study (JCOG0607). Gastric Cancer. 2018; 21:114–123.
Article3. Zhang X, Li M, Chen S, et al. Endoscopic screening in Asian countries is associated with reduced gastric cancer mortality: a meta-analysis and systematic review. Gastroenterology. 2018; 155:347–354.
Article4. Yao K. The endoscopic diagnosis of early gastric cancer. Ann Gastroenterol. 2013; 26:11–22.5. Yao K, Uedo N, Muto M, et al. Development of an e-learning system for the endoscopic diagnosis of early gastric cancer: an international multicenter randomized controlled trial. EBioMedicine. 2016; 9:140–147.
Article6. Nakanishi H, Doyama H, Ishikawa H, et al. Evaluation of an e-learning system for diagnosis of gastric lesions using magnifying narrow-band imaging: a multicenter randomized controlled study. Endoscopy. 2017; 49:957–967.7. Kato M, Uedo N, Nagahama T, et al. Self-study of the non-extension sign in an e-learning program improves diagnostic accuracy of invasion depth of early gastric cancer. Endosc Int Open. 2019; 7:E871–E882.8. Yao K, Uedo N, Muto M, et al. Development of an e-learning system for teaching endoscopists how to diagnose early gastric cancer: basic principles for improving early detection. Gastric Cancer. 2017; 20(Suppl 1):28–38.
Article9. Yao K, Doyama H, Tsuji S. Endoscopic characterization of gastric lesions and resection strategy. In : Testoni PA, Inoue H, Wallace MB, editors. Gastrointestinal and pancreatico-biliary diseases: advanced diagnostic and therapeutic endoscopy. Springer;2021. p. 151–170.10. Yoshida N, Doyama H, Yano T, et al. Early gastric cancer detection in high-risk patients: a multicentre randomised controlled trial on the effect of second-generation narrow band imaging. Gut. 2021; 70:67–75.
Article11. Yao K, Anagnostopoulos GK, Ragunath K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy. 2009; 41:462–467.
Article12. Yao K, Uedo N, Kamada T, et al. Guidelines for endoscopic diagnosis of early gastric cancer. Dig Endosc. 2020; 32:663–698.
Article13. Ezoe Y, Muto M, Uedo N, et al. Magnifying narrowband imaging is more accurate than conventional white-light imaging in diagnosis of gastric mucosal cancer. Gastroenterology. 2011; 141:2017–2025.
Article14. Nagahama T, Yao K, Uedo N, et al. Delineation of the extent of early gastric cancer by magnifying narrow-band imaging and chromoendoscopy: a multicenter randomized controlled trial. Endoscopy. 2018; 50:566–576.
Article15. Muto M, Yao K, Kaise M, et al. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G). Dig Endosc. 2016; 28:379–393.
Article16. Yao K, Doyama H, Gotoda T, et al. Diagnostic performance and limitations of magnifying narrow-band imaging in screening endoscopy of early gastric cancer: a prospective multicenter feasibility study. Gastric Cancer. 2014; 17:669–679.
Article17. Nagahama T, Yao K, Imamura K, et al. Diagnostic performance of conventional endoscopy in the identification of submucosal invasion by early gastric cancer: the "non-extension sign" as a simple diagnostic marker. Gastric Cancer. 2017; 20:304–313.
Article18. Mabe K, Yao K, Nojima M, et al. An educational intervention to improve the endoscopist's ability to correctly diagnose small gastric lesions using magnifying endoscopy with narrow-band imaging. Ann Gastroenterol. 2014; 27:149–155.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoscopic Resection of Undifferentiated Early Gastric Cancer
- Endoscopic Treatment of Stomach Cancer
- Endoscopic Resection of Early Gastric Cancer in Korea: Recent Results and Future Directions
- Endoscopic Submucosal Dissection in the Treatment of Patients With Papillary Early Gastric Cancer
- Endoscopic Resection of Early Gastric Cancer