Brain Tumor Res Treat.
2024 Apr;12(2):141-147. 10.14791/btrt.2024.0017.
Excessively Delayed Radiation Changes After Proton Beam Therapy for Brain Tumors: Report of Two Cases
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University College of Medicine, Seoul, Korea
- 2Departments of Radiation Oncology, National Cancer Center, Goyang, Korea
- 3Departments of Pathology, National Cancer Center, Goyang, Korea
- 4Department of Cancer Control, National Cancer Center, Graduate School of Cancer Science and Policy, Goyang, Korea
- KMID: 2555874
- DOI: http://doi.org/10.14791/btrt.2024.0017
Abstract
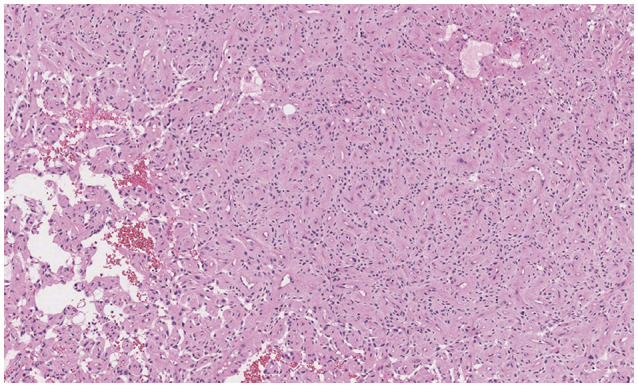
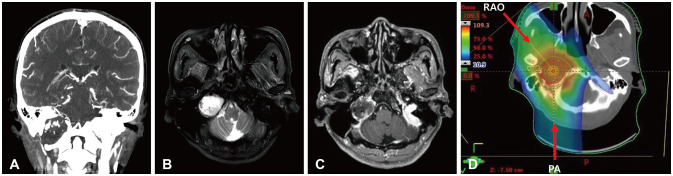
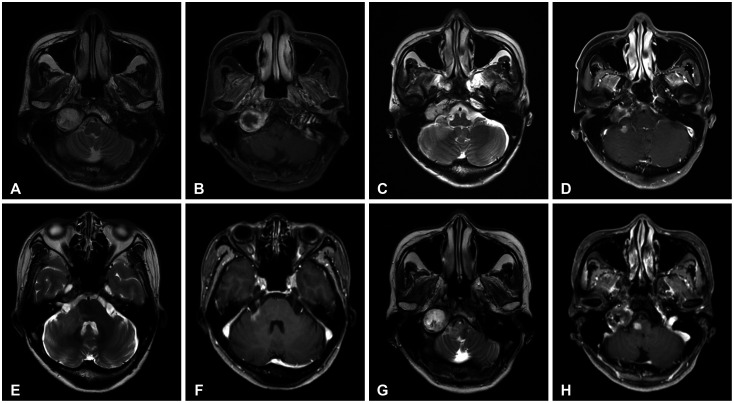
- Delayed cerebral necrosis is a well-known complication of radiation therapy (RT). Because of its irreversible nature, it should be avoided if possible, but avoidance occurs at the expense of potentially compromised tumor control, despite the use of the modern advanced technique of conformal RT that minimizes radiation to normal brain tissue. Risk factors for radiation-induced cerebral necrosis include a higher dose per fraction, larger treatment volume, higher cumulative dose, and shorter time interval (for re-irradiation). The same principle can be applied to proton beam therapy (PBT) to avoid delayed cerebral necrosis. However, conversion of PBT radiation energy into conventional RT is still short of clinical support, compared to conventional RT. Herein, we describe two patients with excessively delayed cerebral necrosis after PBT, in whom follow-up MRI showed no RT-induced changes prior to 3 years after treatment. One patient developed radiation necrosis at 4 years after PBT to the resection cavity of an astroblastoma, and the other developed brainstem necrosis that became symptomatic 6 months after its first appearance on the 3-year follow-up brain MRI. We also discuss possible differences between radiation changes after PBT versus conventional RT.
Keyword
Figure
Reference
-
1. Rahmathulla G, Marko NF, Weil RJ. Cerebral radiation necrosis: a review of the pathobiology, diagnosis and management considerations. J Clin Neurosci. 2013; 20:485–502. PMID: 23416129.2. Gwak HS, Yoo HJ, Youn SM, Lee DH, Kim MS, Rhee CH. Radiosurgery for recurrent brain metastases after whole-brain radiotherapy: factors affecting radiation-induced neurological dysfunction. J Korean Neurosurg Soc. 2009; 45:275–283. PMID: 19516944.3. Dritschilo A, Bruckman JE, Cassady JR, Belli JA. Tolerance of brain to multiple courses of radiation therapy. I. Clinical experiences. Br J Radiol. 1981; 54:782–786. PMID: 7296205.4. Tanaka M, Ino Y, Nakagawa K, Tago M, Todo T. High-dose conformal radiotherapy for supratentorial malignant glioma: a historical comparison. Lancet Oncol. 2005; 6:953–960. PMID: 16321763.5. Andruska N, Kennedy WR, Bonestroo L, Anderson R, Huang Y, Robinson CG, et al. Dosimetric predictors of symptomatic radiation necrosis after five-fraction radiosurgery for brain metastases. Radiother Oncol. 2021; 156:181–187. PMID: 33310010.6. Pennybacker J, Russell DS. Necrosis of the brain due to radiation therapy; clinical and pathological observations. J Neurol Neurosurg Psychiatry. 1948; 11:183–198. PMID: 18878024.7. Furuse M, Nonoguchi N, Kawabata S, Miyatake S, Kuroiwa T. Delayed brain radiation necrosis: pathological review and new molecular targets for treatment. Med Mol Morphol. 2015; 48:183–190. PMID: 26462915.8. Munier S, Ginalis EE, Patel NV, Danish S, Hanft S. Radiation necrosis in intracranial lesions. Cureus. 2020; 12:e7603. PMID: 32399337.9. Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys. 1980; 6:1215–1228. PMID: 7007303.10. Ang KK, Price RE, Stephens LC, Jiang GL, Feng Y, Schultheiss TE, et al. The tolerance of primate spinal cord to re-irradiation. Int J Radiat Oncol Biol Phys. 1993; 25:459–464. PMID: 8436524.11. Hazuka MB, Kinzie JJ. Brain metastases: results and effects of re-irradiation. Int J Radiat Oncol Biol Phys. 1988; 15:433–437. PMID: 2841266.12. Wen J, Tan AP, Yong HRC, Wong YLJ. Delayed radiation necrosis and evolution of its imaging features over time: an illustrative case report. J Oncol Pract. 2018; 14:754–756. PMID: 30537451.13. Giglio P, Gilbert MR. Cerebral radiation necrosis. Neurologist. 2003; 9:180–188. PMID: 12864928.14. Fan X, Huang Y, Xu P, Min Y, Li J, Feng M, et al. Dosimetric analysis of radiation-induced brainstem necrosis for nasopharyngeal carcinoma treated with IMRT. BMC Cancer. 2022; 22:178. PMID: 35177030.15. Niemierko A, Schuemann J, Niyazi M, Giantsoudi D, Maquilan G, Shih HA, et al. Brain necrosis in adult patients after proton therapy: is there evidence for dependency on linear energy transfer? Int J Radiat Oncol Biol Phys. 2021; 109:109–119. PMID: 32911019.16. Vogel J, Grewal A, O’Reilly S, Lustig R, Kurtz G, Minturn JE, et al. Risk of brainstem necrosis in pediatric patients with central nervous system malignancies after pencil beam scanning proton therapy. Acta Oncol. 2019; 58:1752–1756. PMID: 31512931.17. Lühr A, von Neubeck C, Krause M, Troost EGC. Relative biological effectiveness in proton beam therapy–current knowledge and future challenges. Clin Transl Radiat Oncol. 2018; 9:35–41. PMID: 29594249.18. Haas-Kogan D, Indelicato D, Paganetti H, Esiashvili N, Mahajan A, Yock T, et al. National Cancer Institute Workshop on Proton Therapy for Children: considerations regarding brainstem injury. Int J Radiat Oncol Biol Phys. 2018; 101:152–168. PMID: 29619963.19. Kralik SF, Ho CY, Finke W, Buchsbaum JC, Haskins CP, Shih CS. Radiation necrosis in pediatric patients with brain tumors treated with proton radiotherapy. AJNR Am J Neuroradiol. 2015; 36:1572–1578. PMID: 26138138.20. Takahashi M, Mizumoto M, Oshiro Y, Kino H, Akutsu H, Nakai K, et al. Risk factors for radiation necrosis and local recurrence after proton beam therapy for skull base chordoma or chondrosarcoma. Cancers (Basel). 2023; 15:5687. PMID: 38067389.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Radiation Therapy against Pediatric Malignant Central Nervous System Tumors : Embryonal Tumors and Proton Beam Therapy
- Proton Therapy Review: Proton Therapy from a Medical
- Proton therapy in pediatric brain tumors
- Recent Updates on Radiation Therapy for Pediatric Optic Pathway Glioma
- A Pilot Study of the Scanning Beam Quality Assurance Using Machine Log Files in Proton Beam Therapy