Ann Surg Treat Res.
2024 May;106(5):284-295. 10.4174/astr.2024.106.5.284.
Effects of transcription factor SOX11 on the biological behavior of neuroblastoma cell and potential regulatory mechanism
- Affiliations
-
- 1Department of Pediatric Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 2Department of Pathology, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 3Department of Pediatric Surgery, Qilu Hospital of Shandong University, Jinan, China
- KMID: 2555725
- DOI: http://doi.org/10.4174/astr.2024.106.5.284
Abstract
- Purpose
This study aimed to analyze the expression and prognosis of SRY-box transcription factor 11 (SOX11) in neuroblastoma (NB), as well as the biological function and potential regulatory mechanism of SOX11 in NB.
Methods
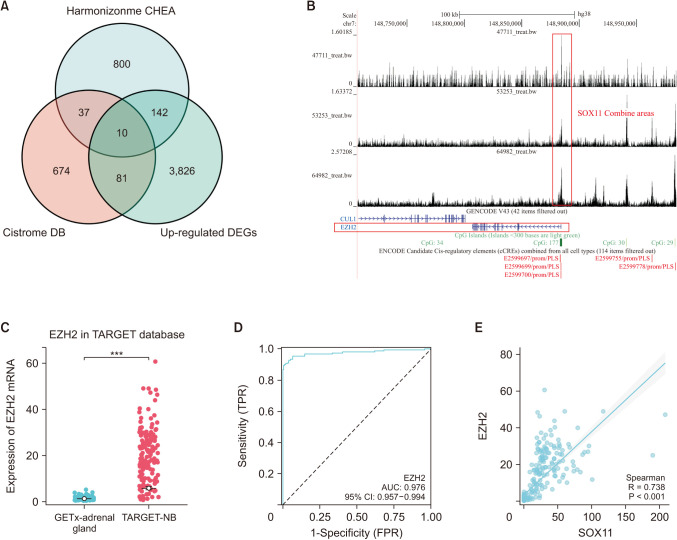
Public RNA sequencing was used to detect the expression level of SOX11. The Kaplan-Meier curve and hazard ratios (HR) were used to determine the prognostic value of SOX11 in NB. Functional analyses were performed using CCK8, wound healing assay, and transwell invasion assay. Finally, the potential target genes of SOX11 were predicted by Harmonizonme (Ma'ayan Laboratory) and Cistrome Data Browser (Cistrome Project) database to explore the potential molecular mechanism of SOX11 in NB.
Results
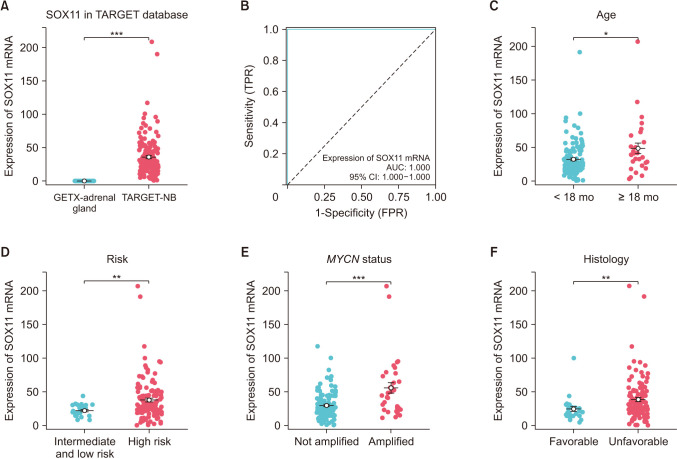
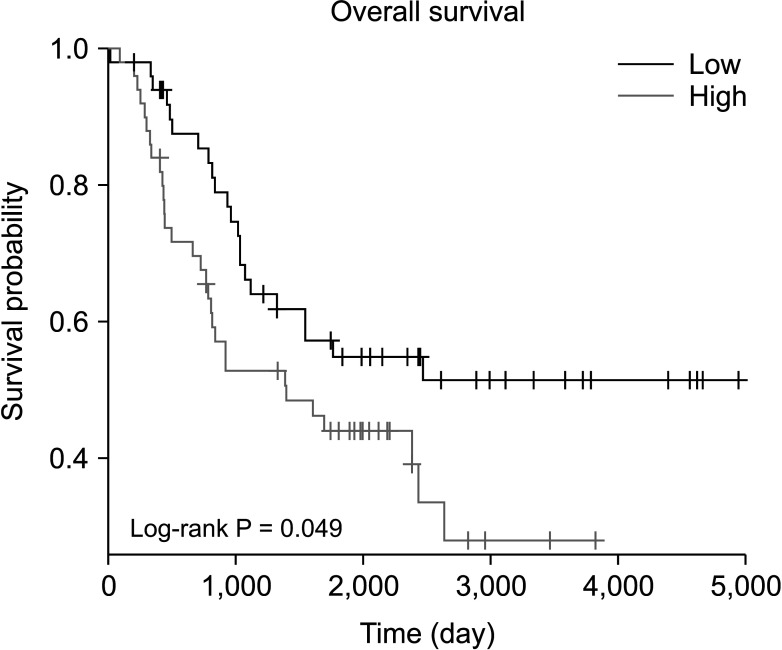
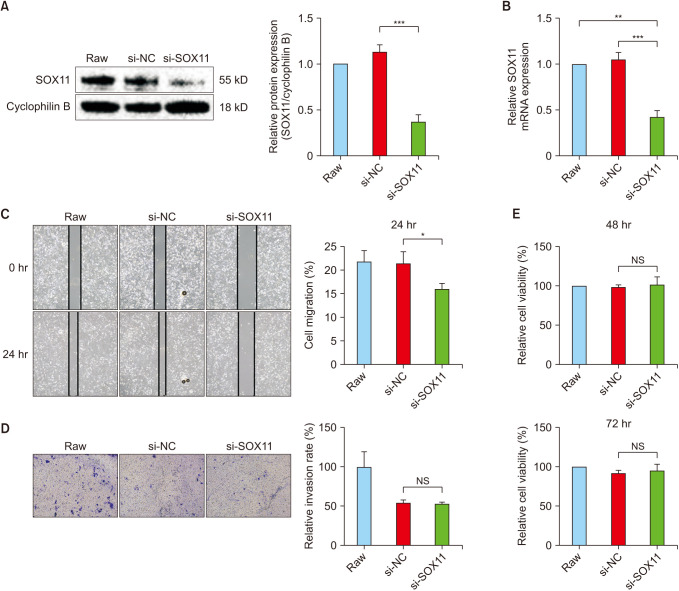
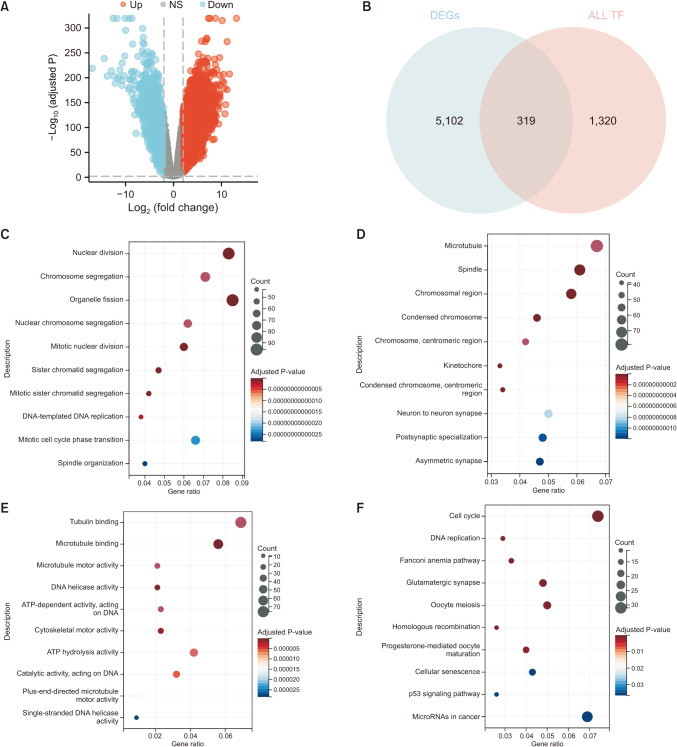
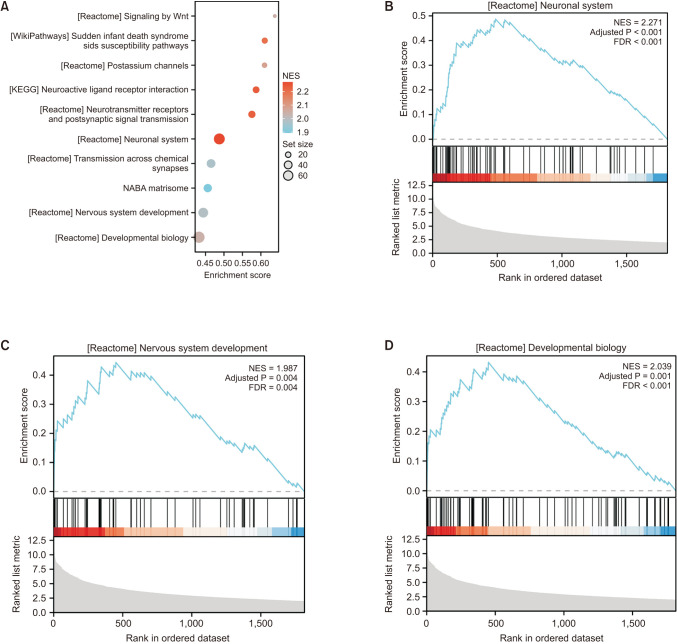
Compared with normal adrenal tissue, the expression of SOX11 in NB tissue was significantly upregulated. The Kaplan-Meier curve showed that high expression of SOX11 was associated with poor prognosis in children with NB (HR, 1.719; P = 0.049). SOX11 knockdown suppressed the migration capacity of SK-N-SH cells but did not affect proliferation and invasion capacity. Enhancer of zeste homolog 2 (EZH2) may be a potential downstream target gene for the transcription factor SOX11 to play a role in NB.
Conclusion
The transcription factor SOX11 was significantly upregulated in NB. SOX11 knockdown suppressed the migration capacity of NB cell SK-N-SH. SOX11 may promote the progression of NB by targeting EZH2.
Keyword
Figure
Reference
-
1. Jansky S, Sharma AK, Körber V, Quintero A, Toprak UH, Wecht EM, et al. Single-cell transcriptomic analyses provide insights into the developmental origins of neuroblastoma. Nat Genet. 2021; 53:683–693. PMID: 33767450.2. Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010; 362:2202–2211. PMID: 20558371.3. Liang SW, Chen G, Luo YG, Chen P, Gu JH, Xu QQ, et al. Nomogram for predicting overall survival in children with neuroblastoma based on SEER database. Ann Surg Treat Res. 2020; 99:118–126. PMID: 32802817.4. Aygun N. Biological and genetic features of neuroblastoma and their clinical importance. Curr Pediatr Rev. 2018; 14:73–90. PMID: 29380702.5. Zafar A, Wang W, Liu G, Wang X, Xian W, McKeon F, et al. Molecular targeting therapies for neuroblastoma: progress and challenges. Med Res Rev. 2021; 41:961–1021. PMID: 33155698.6. Yang Z, Jiang S, Lu C, Ji T, Yang W, Li T, et al. SOX11: friend or foe in tumor prevention and carcinogenesis? Ther Adv Med Oncol. 2019; 11:1758835919853449. PMID: 31210798.7. Tsang SM, Oliemuller E, Howard BA. Regulatory roles for SOX11 in development, stem cells and cancer. Semin Cancer Biol. 2020; 67(Pt 1):3–11. PMID: 32574812.8. Orqueda AJ, Gatti CR, Ogara MF, Falzone TL. SOX-11 regulates LINE-1 retrotransposon activity during neuronal differentiation. FEBS Lett. 2018; 592:3708–3719. PMID: 30276805.9. Wang J, Wang Z, Lin W, Han Q, Yan H, Yao W, et al. LINC01296 promotes neuroblastoma tumorigenesis via the NCL-SOX11 regulatory complex. Mol Ther Oncolytics. 2022; 24:834–848. PMID: 35317520.10. Decaesteker B, Louwagie A, Loontiens S, De Vloed F, Bekaert SL, Roels J, et al. SOX11 regulates SWI/SNF complex components as member of the adrenergic neuroblastoma core regulatory circuitry. Nat Commun. 2023; 14:1267. PMID: 36882421.11. Smith JL, Ries RE, Hylkema T, Alonzo TA, Gerbing RB, Santaguida MT, et al. Comprehensive transcriptome profiling of cryptic CBFA2T3-GLIS2 fusion-positive AML defines novel therapeutic options: a COG and TARGET Pediatric AML Study. Clin Cancer Res. 2020; 26:726–737. PMID: 31719049.12. GTEx Consortium. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013; 45:580–585. PMID: 23715323.13. Johnston SJ, Carroll JS. Transcription factors and chromatin proteins as therapeutic targets in cancer. Biochim Biophys Acta. 2015; 1855:183–192. PMID: 25721328.14. Huang J, Ji EH, Zhao X, Cui L, Misuno K, Guo M, et al. Sox11 promotes head and neck cancer progression via the regulation of SDCCAG8. J Exp Clin Cancer Res. 2019; 38:138. PMID: 30922366.15. Chang L, Yuan Z, Shi H, Bian Y, Guo R. miR-145 targets the SOX11 3’UTR to suppress endometrial cancer growth. Am J Cancer Res. 2017; 7:2305–2317. PMID: 29218252.16. Xue F, Song X, Zhang S, Niu M, Cui Y, Wang Y, et al. Long non-coding RNA TMPO-AS1 serves as a tumor promoter in pancreatic carcinoma by regulating miR-383-5p/SOX11. Oncol Lett. 2021; 21:255. PMID: 33664819.17. Yang R, Huo Z, Duan Y, Tong W, Zheng Y, Su Y, et al. SOX11 inhibits tumor proliferation and promotes cell adhesion mediated-drug resistance via a CD43 dependent manner in mantle cell lymphoma. Leuk Lymphoma. 2020; 61:2068–2081. PMID: 32449421.18. Liu Z, Zhong Y, Chen YJ, Chen H. SOX11 regulates apoptosis and cell cycle in hepatocellular carcinoma via Wnt/β-catenin signaling pathway. Biotechnol Appl Biochem. 2019; 66:240–246. PMID: 30517979.19. Qu Y, Zhou C, Zhang J, Cai Q, Li J, Du T, et al. The metastasis suppressor SOX11 is an independent prognostic factor for improved survival in gastric cancer. Int J Oncol. 2014; 44:1512–1520. PMID: 24604109.20. Akita N, Okada R, Mukae K, Sugino RP, Takenobu H, Chikaraishi K, et al. Polycomb group protein BMI1 protects neuroblastoma cells against DNA damage-induced apoptotic cell death. Exp Cell Res. 2023; 422:113412. PMID: 36370852.21. Ye Z, Chen D, Zheng R, Chen H, Xu T, Wang C, et al. Curcumin induced G2/M cycle arrest in SK-N-SH neuroblastoma cells through the ROS-mediated p53 signaling pathway. J Food Biochem. 2021; 45:e13888. PMID: 34331475.22. Hussain SS, Faizi S, Rafi K, Simjee SU. Novel Mannich base 3FB3FA8H induces apoptosis by upregulating P53 pathway in neuroblastoma cells. Mol Cell Biochem. 2020; 471:29–39. PMID: 32472321.23. Federmann B, Frauenfeld L, Pertsch H, Borgmann V, Steinhilber J, Bonzheim I, et al. Highly sensitive and specific in situ hybridization assay for quantification of SOX11 mRNA in mantle cell lymphoma reveals association of TP53 mutations with negative and low SOX11 expression. Haematologica. 2020; 105:754–764. PMID: 31296581.24. Hanaki S, Shimada M. Targeting EZH2 as cancer therapy. J Biochem. 2021; 170:1–4. PMID: 33479735.25. Li Z, Takenobu H, Setyawati AN, Akita N, Haruta M, Satoh S, et al. EZH2 regulates neuroblastoma cell differentiation via NTRK1 promoter epigenetic modifications. Oncogene. 2018; 37:2714–2727. PMID: 29507419.26. Bownes LV, Williams AP, Marayati R, Stafman LL, Markert H, Quinn CH, et al. EZH2 inhibition decreases neuroblastoma proliferation and in vivo tumor growth. PLoS One. 2021; 16:e0246244. PMID: 33690617.27. Wang L, Chen C, Song Z, Wang H, Ye M, Wang D, et al. EZH2 depletion potentiates MYC degradation inhibiting neuroblastoma and small cell carcinoma tumor formation. Nat Commun. 2022; 13:12. PMID: 35013218.28. Wang C, Liu Z, Woo CW, Li Z, Wang L, Wei JS, et al. EZH2 Mediates epigenetic silencing of neuroblastoma suppressor genes CASZ1, CLU, RUNX3, and NGFR. Cancer Res. 2012; 72:315–324. PMID: 22068036.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Calibrating Thresholds to Improve the Detection Accuracy of Putative Transcription Factor Binding Sites
- Overexpression of neurogenin1 induces neurite outgrowth in F11 neuroblastoma cells
- Regulatory Role of Hypoxia Inducible Factor in the Biological Behavior of Nucleus Pulposus Cells
- Role of HIF1α Regulatory Factors in Stem Cells
- Transcriptional Network Controlling Endochondral Ossification