Korean J Physiol Pharmacol.
2024 May;28(3):275-284. 10.4196/kjpp.2024.28.3.275.
Development and assessment of nano drug delivery systems for combined delivery of rosuvastatin and ezetimibe
- Affiliations
-
- 1Department of Zoology, Faculty of Science, Al-Azhar University, Cairo 11651, Egypt
- 2Department of Zoology, Faculty of Science, Zagazig University, Zagazig 44519, Egypt
- 3Department of Zoology, Faculty of Science, Mansoura University, Mansoura 35516, Egypt
- 4Department of Pharmaceutics, Faculty of Pharmacy, Zagazig University, Zagazig 44519, Egypt
- 5Department of Pharmaceutics, Faculty of Pharmacy, Badr University in Cairo, Cairo 11829, Egypt
- KMID: 2555669
- DOI: http://doi.org/10.4196/kjpp.2024.28.3.275
Abstract
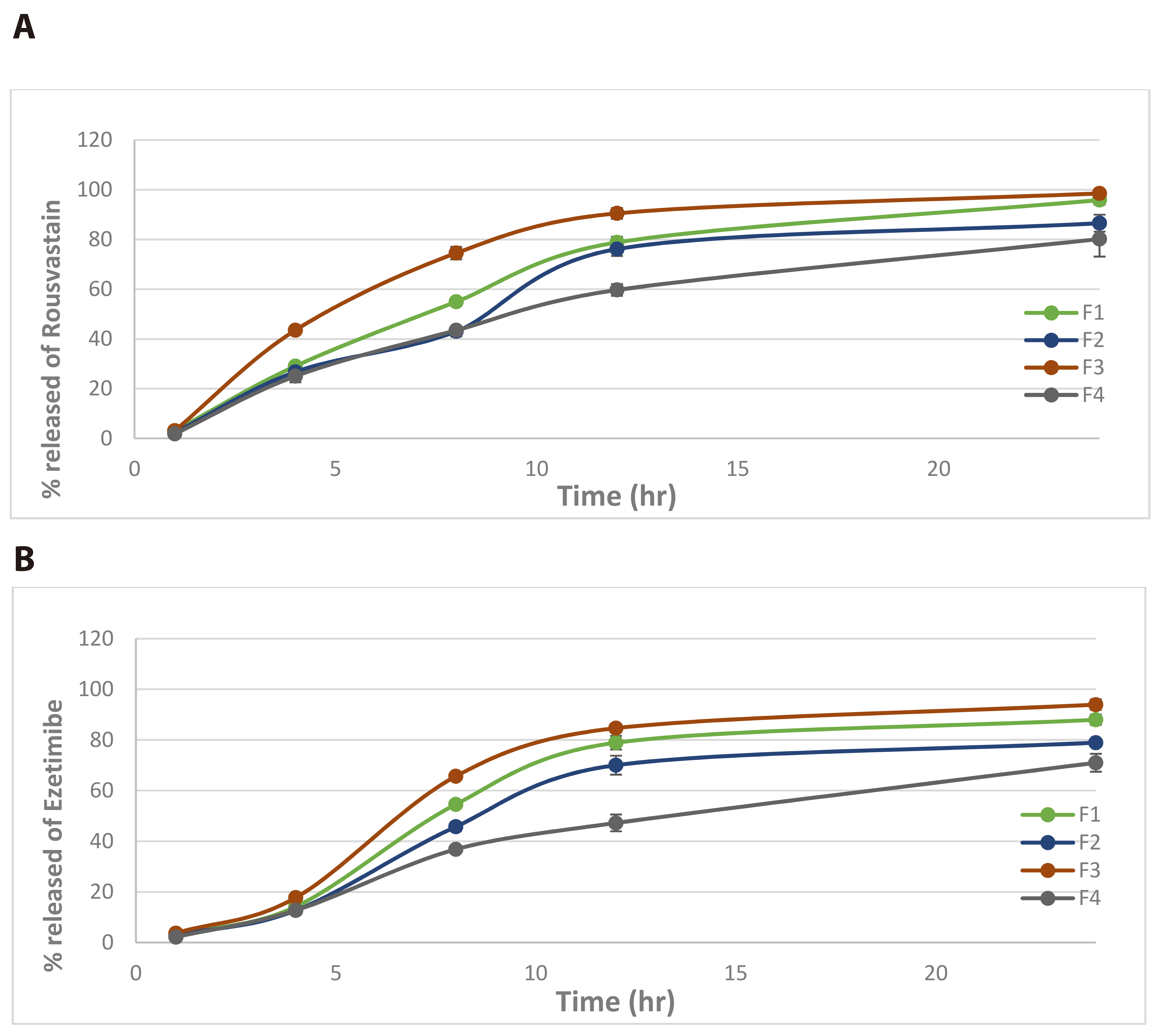
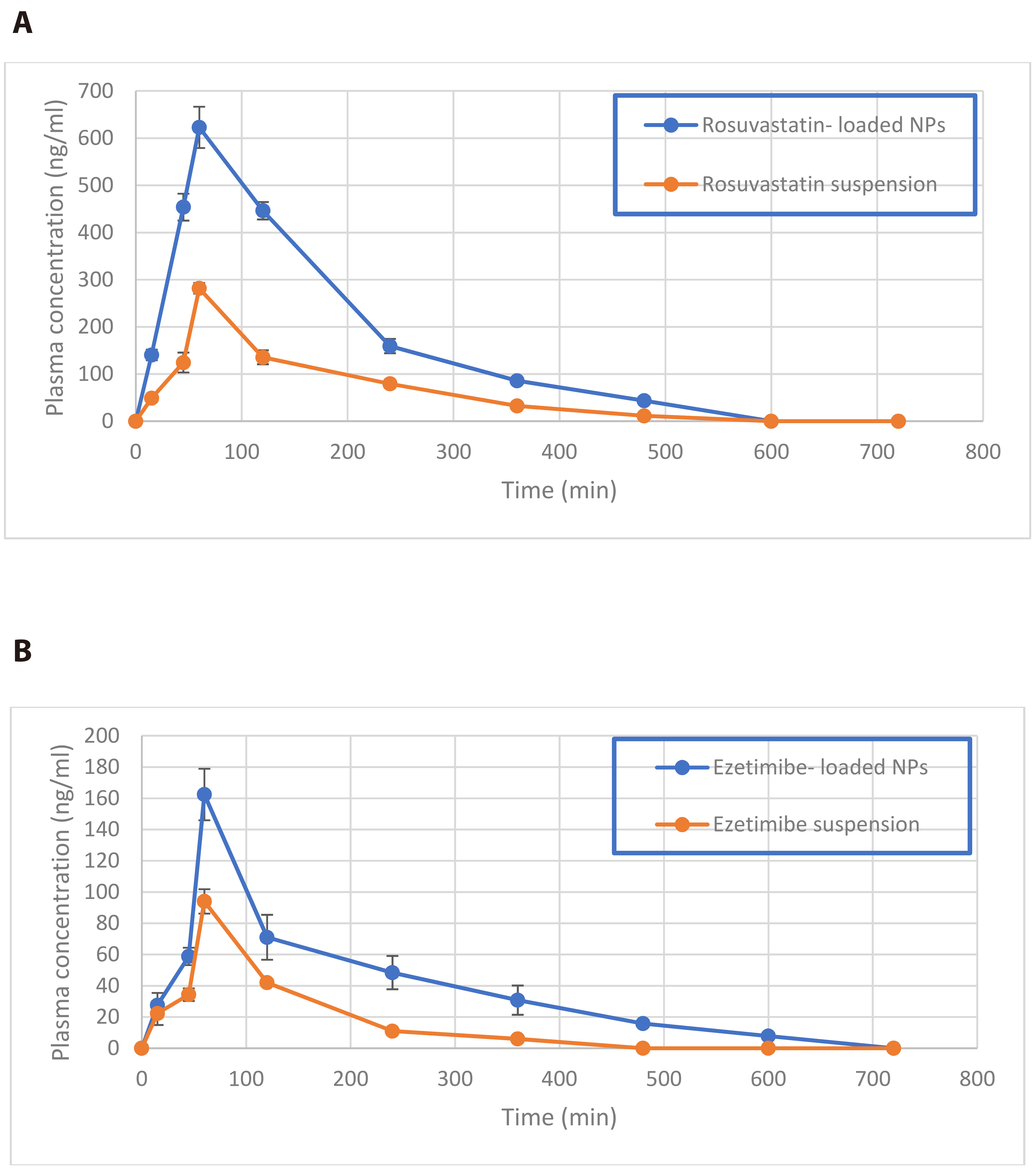
- Worldwide, cardiovascular disease is the main cause of death, which accordingly increased by hyperlipidemia. Hyperlipidemia therapy can include lifestyle changes and medications to control cholesterol levels. Statins are the medications of the first choice for dealing with lipid abnormalities. Rosuvastatin founds to control high lipid levels by hindering liver production of cholesterol and to achieve the targeted levels of low-density lipoprotein cholesterol, another lipid lowering agents named ezetimibe may be used as an added therapy. Both rosuvastatin and ezetimibe have low bioavailability which will stand as barrier to decrease cholesterol levels, because of such depictions, formulations of this combined therapy in nanotechnology will be of a great assistance. Our study demonstrated preparations of nanoparticles of this combined therapy, showing their physical characterizations, and examined their behavior in laboratory conditions and vivo habitation. The mean particle size was uniform, polydispersity index and zeta potential of formulations were found to be in the ranges of (0.181–0.72) and (–13.4 to –6.24), respectively. Acceptable limits of entrapment efficiency were affirmed with appearance of spherical and uniform nanoparticles. In vitro testing showed a sustained release of drug exceeded 90% over 24 h. In vivo study revealed an enhanced dissolution and bioavailability from loaded nanoparticles, which was evidenced by calculated pharmacokinetic parameters using triton for hyperlipidemia induction. Stability studies were performed and assured that the formulations are kept the same up to one month. Therefore, nano formulations is a suitable transporter for combined therapy of rosuvastatin and ezetimibe with improvement in their dissolution and bioavailability.
Keyword
Figure
Reference
-
1. Nelson RH. 2013; Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care. 40:195–211. DOI: 10.1016/j.pop.2012.11.003. PMID: 23402469. PMCID: PMC3572442.
Article2. Skolnik N, Jaffa FM, Kalyani RR, Johnson E, Shubrook JH. 2017; Reducing CV risk in diabetes: an ADA update. J Fam Pract. 66:300–308.3. Meena K, Misra A, Vikram N, Ali S, Pandey RM, Luthra K. 2011; Cholesterol ester transfer protein and apolipoprotein E gene polymorphisms in hyperlipidemic Asian Indians in North India. Mol Cell Biochem. 352:189–196. DOI: 10.1007/s11010-011-0753-1. PMID: 21380728.
Article4. Alissa EM, Ferns GA. 2017; Dietary fruits and vegetables and cardiovascular diseases risk. Crit Rev Food Sci Nutr. 57:1950–1962.
Article5. Keshetty V, Pabba S, Gudipati R, Kandukuri JM, Allenki V. 2009; Antihyperlipidemic activity of methanolic extract of Garlic (Alliumsativum L.) in Triton X-100 induced hyperlipidemic rats. J Pharm Res. 2:777–780.6. Pushpa I, Jayachitra J. 2015; Hypolipidemic and antioxidant activity of phoenix dactylifera L. in albino wistar rats. World J Pharm Pharm Sci. 4:790–798.7. Patel AY, Pillarisetti J, Marr J, Vacek JL. 2013; Ezetimibe in combination with a statin does not reduce all-cause mortality. J Clin Med Res. 5:275–280. DOI: 10.4021/jocmr1371w. PMID: 23864916. PMCID: PMC3712882.
Article8. Feingold KR, Chait A. 2023; Approach to patients with elevated low-density lipoprotein cholesterol levels. Best Pract Res Clin Endocrinol Metab. 37:101658. DOI: 10.1016/j.beem.2022.101658. PMID: 35487874.
Article9. Kostapanos MS, Milionis HJ, Elisaf MS. 2010; Rosuvastatin-associated adverse effects and drug-drug interactions in the clinical setting of dyslipidemia. Am J Cardiovasc Drugs. 10:11–28. DOI: 10.2165/13168600-000000000-00000. PMID: 20104931.
Article10. Ballantyne CM, Houri J, Notarbartolo A, Melani L, Lipka LJ, Suresh R, Sun S, LeBeaut AP, Sager PT, Veltri EP. Ezetimibe Study Group. 2003; Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation. 107:2409–2415. DOI: 10.1161/01.CIR.0000068312.21969.C8. PMID: 12719279.
Article11. Rashid R, Kim DW, Din FU, Mustapha O, Yousaf AM, Park JH, Kim JO, Yong CS, Choi HG. 2015; Effect of hydroxypropylcellulose and Tween 80 on physicochemical properties and bioavailability of ezetimibe-loaded solid dispersion. Carbohydr Polym. 130:26–31. DOI: 10.1016/j.carbpol.2015.04.071. PMID: 26076597.
Article12. Karanam SR, Katakam P, Chandu BR, Hwisa NT, Adiki SK. 2014; Simultaneous determination of ezetimibe and simvastatin in rat plasma by stable-isotope dilution LC-ESI-MS/MS and its application to a pharmacokinetic study. J Pharm Anal. 4:286–294. DOI: 10.1016/j.jpha.2013.08.002. PMID: 29403892. PMCID: PMC5761217.
Article13. Din FU, Zeb A, Shah KU, Rehman ZU. 2019; Development, in-vitro and in-vivo evaluation of ezetimibe-loaded solid lipid nanoparticles and their comparison with marketed product. J Drug Deliv Sci Technol. 51:583–590. DOI: 10.1016/j.jddst.2019.02.026.
Article14. Suchy D, Łabuzek K, Stadnicki A, Okopień B. 2011; Ezetimibe--a new approach in hypercholesterolemia management. Pharmacol Rep. 63:1335–1348. DOI: 10.1016/S1734-1140(11)70698-3. PMID: 22358082.15. Montelione N, Loreni F, Nenna A, Catanese V, Scurto L, Ferrisi C, Jawabra M, Gabellini T, Codispoti FA, Spinelli F, Chello M, Stilo F. 2023; Tissue engineering and targeted drug delivery in cardiovascular disease: the role of polymer nanocarrier for statin therapy. Biomedicines. 11:798. DOI: 10.3390/biomedicines11030798. PMID: 36979777. PMCID: PMC10045667.
Article16. Orive G, Gascón AR, Hernández RM, Domínguez-Gil A, Pedraz JL. 2004; Techniques: new approaches to the delivery of biopharmaceuticals. Trends Pharmacol Sci. 25:382–387. DOI: 10.1016/j.tips.2004.05.006. PMID: 15219981.
Article17. Cheng LC, Jiang X, Wang J, Chen C, Liu RS. 2013; Nano-bio effects: interaction of nanomaterials with cells. Nanoscale. 5:3547–3569. DOI: 10.1039/c3nr34276j. PMID: 23532468.
Article18. Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, Habtemariam S, Shin HS. 2018; Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 16:71. DOI: 10.1186/s12951-018-0392-8. PMID: 30231877. PMCID: PMC6145203.
Article19. Surendiran A, Sandhiya S, Pradhan SC, Adithan C. 2009; Novel applications of nanotechnology in medicine. Indian J Med Res. 130:689–701.20. Fessi H, Puisieux F, Devissaguet JP, Ammoury N, Benita S. 1989; Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int J Pharm. 55:R1–R4. DOI: 10.1016/0378-5173(89)90281-0.
Article21. Kiani A, Fathi M, Ghasemi SM. 2017; Production of novel vitamin D3 loaded lipid nanocapsules for milk fortification. Int J Food Prop. 20:2466–2476. DOI: 10.1080/10942912.2016.1240690.
Article22. Abdel-Mottaleb MM, Neumann D, Lamprecht A. 2010; In vitro drug release mechanism from lipid nanocapsules (LNC). Int J Pharm. 390:208–213. DOI: 10.1016/j.ijpharm.2010.02.001. PMID: 20149853.
Article23. Hussein A, Abdel-Mottaleb MMA, El-assal M, Sammour O. 2020; Novel biocompatible essential oil-based lipid nanocapsules with antifungal properties. J Drug Deliv Sci Technol. 56:101605. DOI: 10.1016/j.jddst.2020.101605.
Article24. Kakkar D, Dumoga S, Kumar R, Chuttani K, Mishra AK. 2015; PEGylated solid lipid nanoparticles: design, methotrexate loading and biological evaluation in animal models. Med Chem Commun. 6:1452–1463. DOI: 10.1039/C5MD00104H.
Article25. Din FU, Mustapha O, Kim DW, Rashid R, Park JH, Choi JY, Ku SK, Yong CS, Kim JO, Choi HG. 2015; Novel dual-reverse thermosensitive solid lipid nanoparticle-loaded hydrogel for rectal administration of flurbiprofen with improved bioavailability and reduced initial burst effect. Eur J Pharm Biopharm. 94:64–72. DOI: 10.1016/j.ejpb.2015.04.019. PMID: 25979136.
Article26. Bae JW, Choi CI, Park SH, Jang CG, Lee SY. 2012; Analytical LC-MS/MS method for ezetimibe and its application for pharmacokinetic study. J Liq Chromatogr Relat Technol. 35:141–152. DOI: 10.1080/10826076.2011.597065.
Article27. Liu Z, Zhang X, Wu H, Li J, Shu L, Liu R, Li L, Li N. 2011; Preparation and evaluation of solid lipid nanoparticles of baicalin for ocular drug delivery system in vitro and in vivo. Drug Dev Ind Pharm. 37:475–481. DOI: 10.3109/03639045.2010.522193. PMID: 21054217.
Article28. Cho JH, Kim YI, Kim DW, Yousaf AM, Kim JO, Woo JS, Yong CS, Choi HG. 2014; Development of novel fast-dissolving tacrolimus solid dispersion-loaded prolonged release tablet. Eur J Pharm Sci. 54:1–7. DOI: 10.1016/j.ejps.2013.12.016. PMID: 24388864.
Article29. Dudhipala NR, Ettireddy SR, Puchakayala GR. 2021; Attenuation of lipid levels in triton induced hyperlipidemia rats through rosuvastatin calcium nanoparticles: pharmacokinetic and pharmacodynamic studies. Chem Phys Lipids. 237:105081. DOI: 10.1016/j.chemphyslip.2021.105081. PMID: 33811848.
Article30. Kim JS, Park JH, Jeong SC, Kim DS, Yousaf AM, Din FU, Kim JO, Yong CS, Youn YS, Oh KT, Jin SG, Choi HG. 2018; Novel revaprazan-loaded gelatin microsphere with enhanced drug solubility and oral bioavailability. J Microencapsul. 35:421–427. DOI: 10.1080/02652048.2018.1515997. PMID: 30136606.
Article31. Nekkanti V, Wang Z, Betageri GV. 2016; Pharmacokinetic evaluation of improved oral bioavailability of valsartan: proliposomes versus self-nanoemulsifying drug delivery system. AAPS PharmSciTech. 17:851–862. DOI: 10.1208/s12249-015-0388-8. PMID: 26381913.
Article32. Azhar Shekoufeh Bahari L, Hamishehkar H. 2016; The impact of variables on particle size of solid lipid nanoparticles and nanostructured lipid carriers; a comparative literature review. Adv Pharm Bull. 6:143–151. DOI: 10.15171/apb.2016.021. PMID: 27478775. PMCID: PMC4961971.
Article33. Tefas LR, Tomuţă I, Achim M, Vlase L. 2015; Development and optimization of quercetin-loaded PLGA nanoparticles by experimental design. Clujul Med. 88:214–223. DOI: 10.15386/cjmed-418. PMID: 26528074. PMCID: PMC4576773.
Article34. Kheradmandnia S, Vasheghani-Farahani E, Nosrati M, Atyabi F. 2010; The effect of process variables on the properties of ketoprofen loaded solid lipid nanoparticles of beeswax and carnauba wax. Iran J Chem Chem Eng. 29:181–187.35. Agrawal YO, Mahajan UB, Agnihotri VV, Nilange MS, Mahajan HS, Sharma C, Ojha S, Patil CR, Goyal SN. 2021; Ezetimibe-loaded nanostructured lipid carrier based formulation ameliorates hyperlipidaemia in an experimental model of high fat diet. Molecules. 26:1485. DOI: 10.3390/molecules26051485. PMID: 33803259. PMCID: PMC7967240.
Article36. Al-Heibshy FNS, Başaran E, Arslan R, Öztürk N, Erol K, Demirel M. 2020; Physicochemical characterization and pharmacokinetic evaluation of rosuvastatin calcium incorporated solid lipid nanoparticles. Int J Pharm. 578:119106. DOI: 10.1016/j.ijpharm.2020.119106. PMID: 32014599.
Article37. Müller RH, Mäder K, Gohla S. 2000; Solid lipid nanoparticles (SLN) for controlled drug delivery - a review of the state of the art. Eur J Pharm Biopharm. 50:161–177. DOI: 10.1016/S0939-6411(00)00087-4. PMID: 10840199.
Article38. Das S, Suresh PK, Desmukh R. 2010; Design of Eudragit RL 100 nanoparticles by nanoprecipitation method for ocular drug delivery. Nanomedicine. 6:318–823. DOI: 10.1016/j.nano.2009.09.002. PMID: 19800990.
Article39. Kumar R, Singh A, Sharma K, Dhasmana D, Garg N, Siril PF. 2020; Preparation, characterization and in vitro cytotoxicity of Fenofibrate and Nabumetone loaded solid lipid nanoparticles. Mater Sci Eng C Mater Biol Appl. 106:110184. DOI: 10.1016/j.msec.2019.110184. PMID: 31753394.
Article40. Kumar PP, Gayatri P, Sunil R, Jaganmohan S, Rao YM. 2012; Atorvastatin loaded solid lipid nanoparticles: formulation, optimization, and in vitro characterization. IOSR J Pharm. 2:23–32. DOI: 10.9790/3013-25102332.
Article41. Sathali H, Abdul A, Nisha N. 2013; Development of solid lipid nanoparticles of rosuvastatin calcium. J Pharm Res. 6:536–548.42. Wissing SA, Kayser O, Müller RH. 2004; Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev. 56:1257–1272. DOI: 10.1016/j.addr.2003.12.002. PMID: 15109768.
Article43. Brazel CS, Huang X. Svenson S, editor. 2004. The cost of optimal drug delivery: reducing and preventing the burst effect in matrix systems. Carrier-based drug delivery. American Chemical Society;p. 267–282. DOI: 10.1021/bk-2004-0879.ch019.
Article44. Hwang I, Park SI, Lee S, Lee B, Yu KS, Jeon JY, Kim MG. 2018; Pharmacokinetics of fixed-dose combination of rosuvastatin 20 mg and ezetimibe 10 mg compared to concurrent administration of individual tablets in healthy Korean subjects. Transl Clin Pharmacol. 26:16–24. DOI: 10.12793/tcp.2018.26.1.16. PMID: 32055543. PMCID: PMC6989225.
Article45. Li J, Yang M, Xu W. 2018; Development of novel rosuvastatin nanostructured lipid carriers for oral delivery in an animal model. Drug Des Devel Ther. 12:2241–2248. DOI: 10.2147/DDDT.S169522. PMID: 30050285. PMCID: PMC6055887.
Article46. Yasim A, Ozbag D, Kilinc M, Ciralik H, Toru H, Gümüşalan Y. 2010; Effect of atorvastatin and ezetimibe treatment on serum lipid profile and oxidative state in rats fed with a high-cholesterol diet. Am J Med Sci. 339:448–452. DOI: 10.1097/MAJ.0b013e3181d4eb71. PMID: 20224310.
Article47. Birnbaum Y, Lin Y, Ye Y, Merla R, Perez-Polo JR, Uretsky BF. 2008; Pretreatment with high-dose statin, but not low-dose statin, ezetimibe, or the combination of low-dose statin and ezetimibe, limits infarct size in the rat. J Cardiovasc Pharmacol Ther. 13:72–79. DOI: 10.1177/1074248407312839. PMID: 18287593.
Article48. Rashid R, Kim DW, Yousaf AM, Mustapha O, Fakhar Ud Din, Park JH, Yong CS, Oh YK, Youn YS, Kim JO, Choi HG. 2015; Comparative study on solid self-nanoemulsifying drug delivery and solid dispersion system for enhanced solubility and bioavailability of ezetimibe. Int J Nanomedicine. 10:6147–6159. DOI: 10.2147/IJN.S91216. PMID: 26491288. PMCID: PMC4598224.49. Saadallah MS, Hamid OA. 2022; Eudragit® L100 nanoparticles for transdermal delivery of rosuvastatin calcium. J Excipients Food Chem. 13:80–93.50. Proudfoot SG. Aulton ME, editor. 1999. Assessment of bioavailability. Pharmaceutics, the science of dosage form design. Churchill Livingstone Edinburgh;p. 174. p. 189.51. McTaggart F. 2003; Comparative pharmacology of rosuvastatin. Atheroscler Suppl. 4:9–14. DOI: 10.1016/S1567-5688(03)00004-7. PMID: 12714032.
Article52. Kosoglou T, Statkevich P, Johnson-Levonas AO, Paolini JF, Bergman AJ, Alton KB. 2005; Ezetimibe: a review of its metabolism, pharmacokinetics and drug interactions. Clin Pharmacokinet. 44:467–494. DOI: 10.2165/00003088-200544050-00002. PMID: 15871634.53. Alhayali A, Selo MA, Ehrhardt C, Velaga S. 2018; Investigation of supersaturation and in vitro permeation of the poorly water soluble drug ezetimibe. Eur J Pharm Sci. 117:147–153. DOI: 10.1016/j.ejps.2018.01.047. PMID: 29408604.
Article54. Din FU, Saleem S, Aleem F, Ahmed R, Huda NU, Ahmed S, Khaleeq N, Shah KU, Ullah I, Zeb A, Aman W. 2018; Advanced colloidal technologies for the enhanced bioavailability of drugs. Cogent Medicine. 5:1480572. DOI: 10.1080/2331205X.2018.1480572.
Article55. Verma R, Kaushik A, Almeer R, Rahman MH, Abdel-Daim MM, Kaushik D. 2021; Improved pharmacodynamic potential of rosuvastatin by self-nanoemulsifying drug delivery system: an in vitro and in vivo evaluation. Int J Nanomedicine. 16:905–924. DOI: 10.2147/IJN.S287665. PMID: 33603359. PMCID: PMC7881784.
Article56. Katsiki N, Theocharidou E, Karagiannis A, Athyros VG, Mikhailidis DP. 2013; Ezetimibe therapy for dyslipidemia: an update. Curr Pharm Des. 19:3107–3114. DOI: 10.2174/13816128113199990314. PMID: 23317398.
Article57. Morris S, Tiller R. 2003; Ezetimibe for hypercholesterolemia. Am Fam Physician. 68:1595–1596.58. Miura S, Saku K. 2008; Beneficial effects of ezetimibe-based therapy in patients with dyslipidemia. J Cardiol. 52:1–6. DOI: 10.1016/j.jjcc.2008.05.001. PMID: 18639771.
Article59. Garcia-Calvo M, Lisnock J, Bull HG, Hawes BE, Burnett DA, Braun MP, Crona JH, Davis HR Jr, Dean DC, Detmers PA, Graziano MP, Hughes M, Macintyre DE, Ogawa A, O'neill KA, Iyer SP, Shevell DE, Smith MM, Tang YS, Makarewicz AM, et al. 2005; The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1). Proc Natl Acad Sci U S A. 102:8132–8137. DOI: 10.1073/pnas.0500269102. PMID: 15928087. PMCID: PMC1149415.
Article60. Neal RC, Jones PH. 2003; Lipid-lowering: can ezetimibe help close the treatment gap? Cleve Clin J Med. 70:777–783. DOI: 10.3949/ccjm.70.9.777. PMID: 14518572.
Article61. Boutari C, Karagiannis A, Athyros VG. 2021; Rosuvastatin and ezetimibe for the treatment of dyslipidemia and hypercholesterolemia. Expert Rev Cardiovasc Ther. 19:575–580. DOI: 10.1080/14779072.2021.1940959. PMID: 34102931.
Article62. Rizzo M, Berneis K, Spinas GA, Rini GB, Kapur NK. 2009; Quantitative and qualitative effects of rosuvastatin on LDL-cholesterol: what is the clinical significance? Int J Clin Pract. 63:478–485. DOI: 10.1111/j.1742-1241.2008.01979.x. PMID: 19222633.
Article63. Pearson TA, Ballantyne CM, Veltri E, Shah A, Bird S, Lin J, Rosenberg E, Tershakovec AM. 2009; Pooled analyses of effects on C-reactive protein and low density lipoprotein cholesterol in placebo-controlled trials of ezetimibe monotherapy or ezetimibe added to baseline statin therapy. Am J Cardiol. 103:369–374. DOI: 10.1016/j.amjcard.2008.09.090. PMID: 19166691.
Article64. Min KL, Park MS, Jung J, Chang MJ, Kim CO. 2017; Comparison of pharmacokinetics and safety of a fixed-dose combination of rosuvastatin and ezetimibe versus separate tablets in healthy subjects. Clin Ther. 39:1799–1810. DOI: 10.1016/j.clinthera.2017.07.038. PMID: 28803122.
Article65. Wirfs MJ. 2021. The APRN and PA's Complete Guide to Prescribing Drug Therapy 2022. Springer Publishing Company.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Corrigendum to: Development and assessment of nano drug delivery systems for combined delivery of rosuvastatin and ezetimibe
- Letter: Comparison of the Efficacy of Rosuvastatin Monotherapy 20 mg with Rosuvastatin 5 mg and Ezetimibe 10 mg Combination Therapy on Lipid Parameters in Patients with Type 2 Diabetes Mellitus (Diabetes Metab J 2019;43:582–9)
- Response: Comparison of the Efficacy of Rosuvastatin Monotherapy 20 mg with Rosuvastatin 5 mg and Ezetimibe 10 mg Combination Therapy on Lipid Parameters in Patients with Type 2 Diabetes Mellitus (Diabetes Metab J 2019;43:582–9)
- Pharmacokinetics of fixed-dose combination of rosuvastatin 20 mg and ezetimibe 10 mg compared to concurrent administration of individual tablets in healthy Korean subjects
- Ultrasound-guided drug delivery in cancer