Korean J Pain.
2024 Apr;37(2):151-163. 10.3344/kjp.23363.
Analgesic and anti-inflammatory effects of galangin: a potential pathway to inhibit transient receptor potential vanilloid 1 receptor activation
- Affiliations
-
- 1Hainan Women and Children’s Medical Center, Haikou, China
- KMID: 2554955
- DOI: http://doi.org/10.3344/kjp.23363
Abstract
- Background
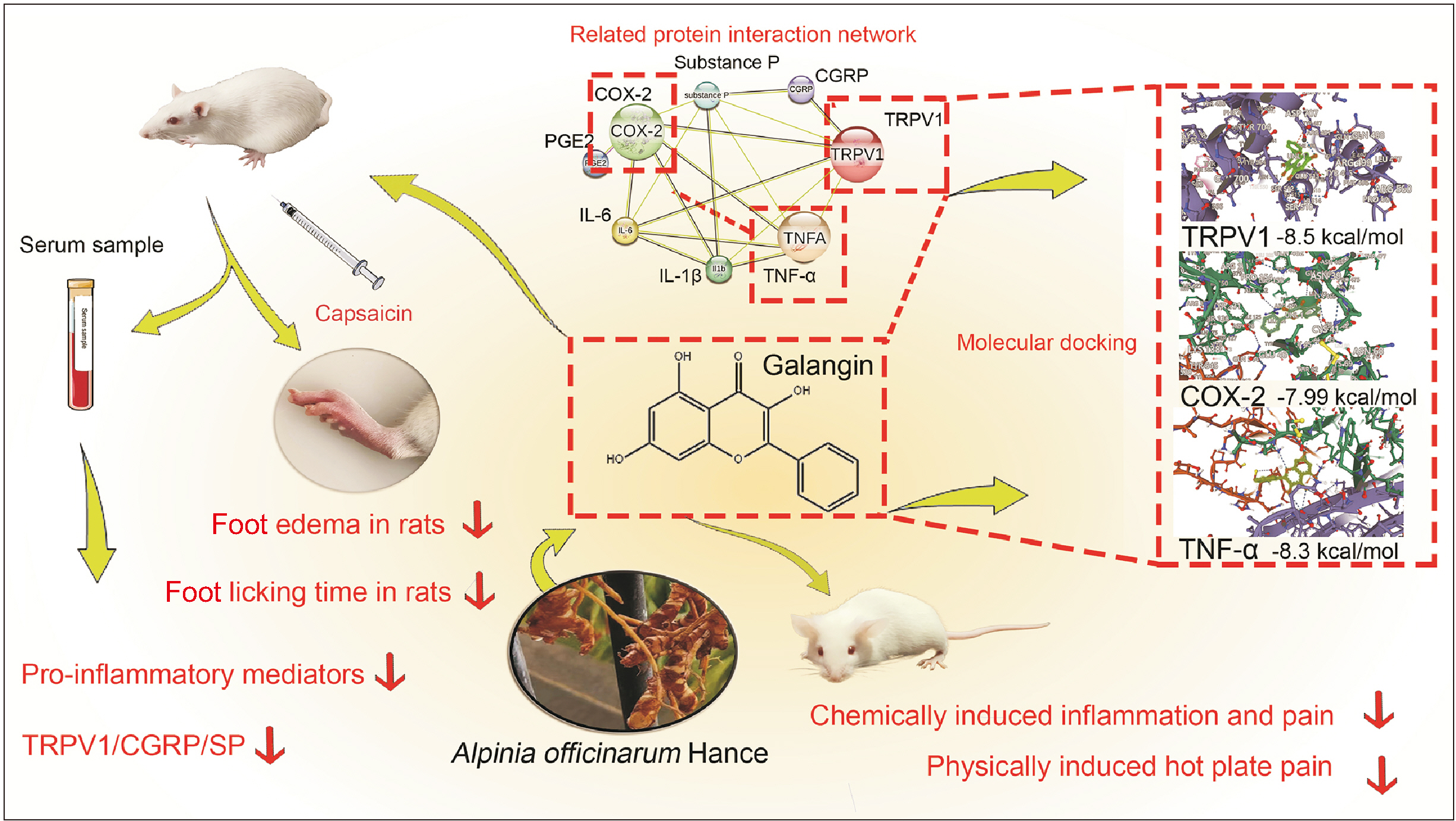
Galangin, commonly employed in traditional Chinese medicine for its diverse medicinal properties, exhibits potential in treating inflammatory pain. Nevertheless, its mechanism of action remains unclear.
Methods
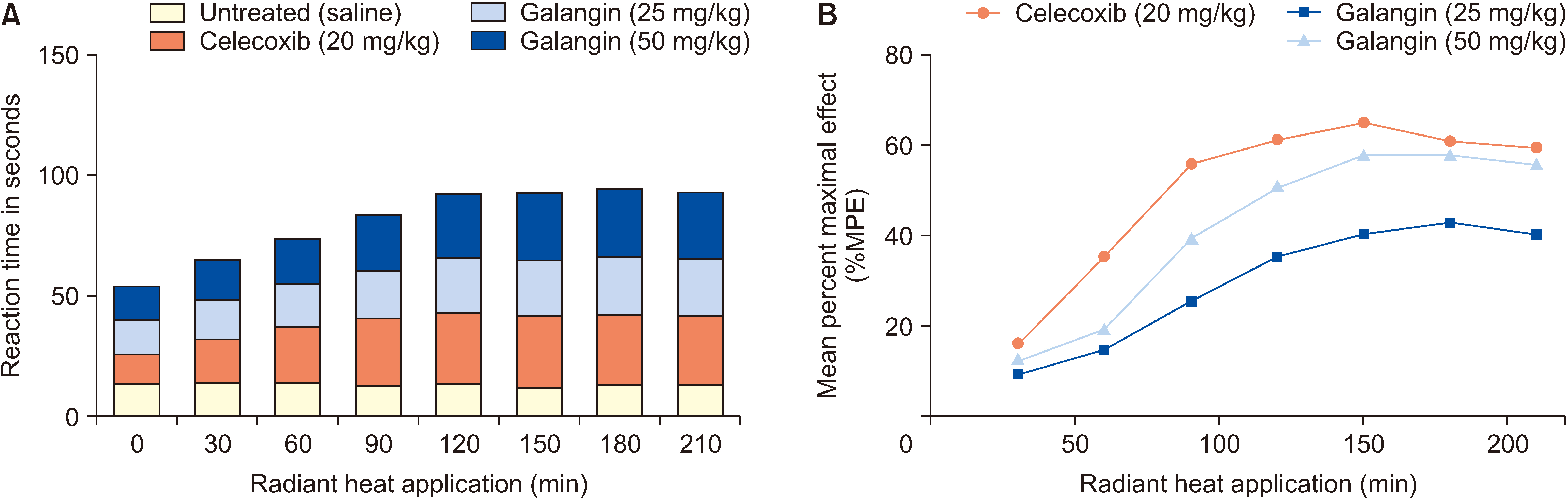
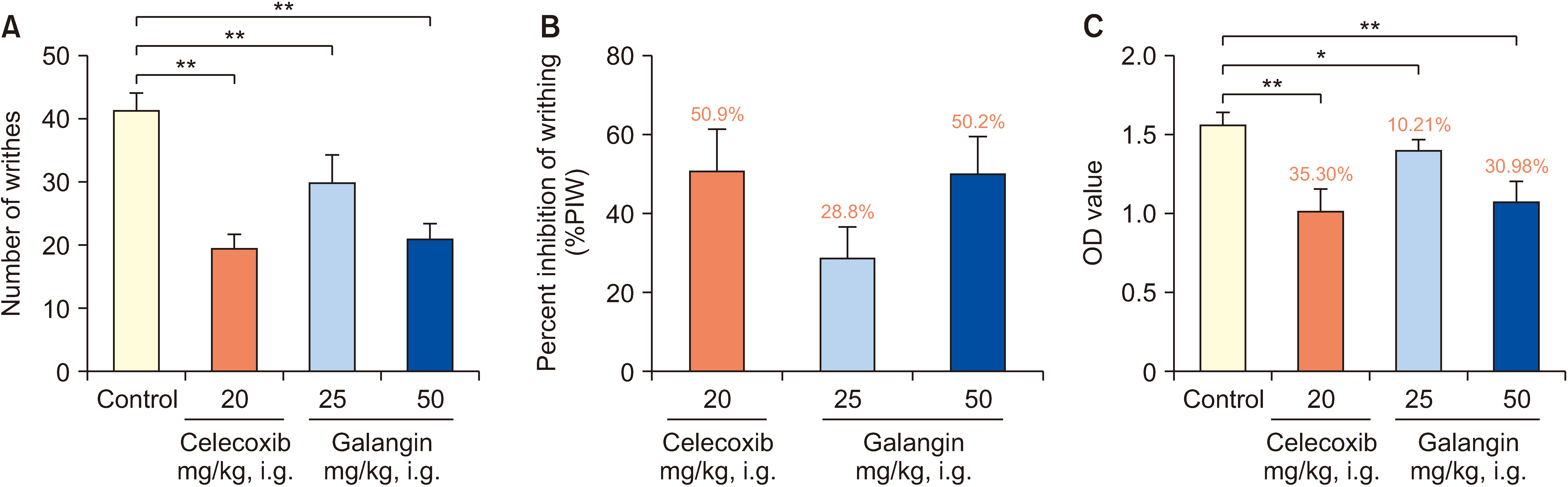
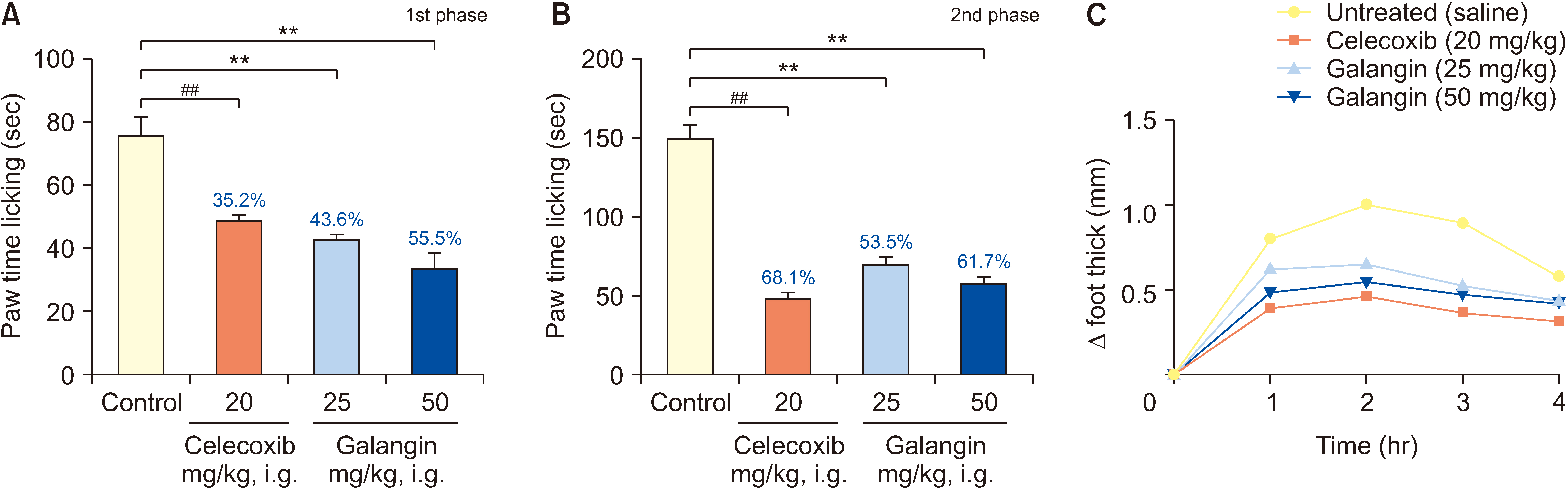
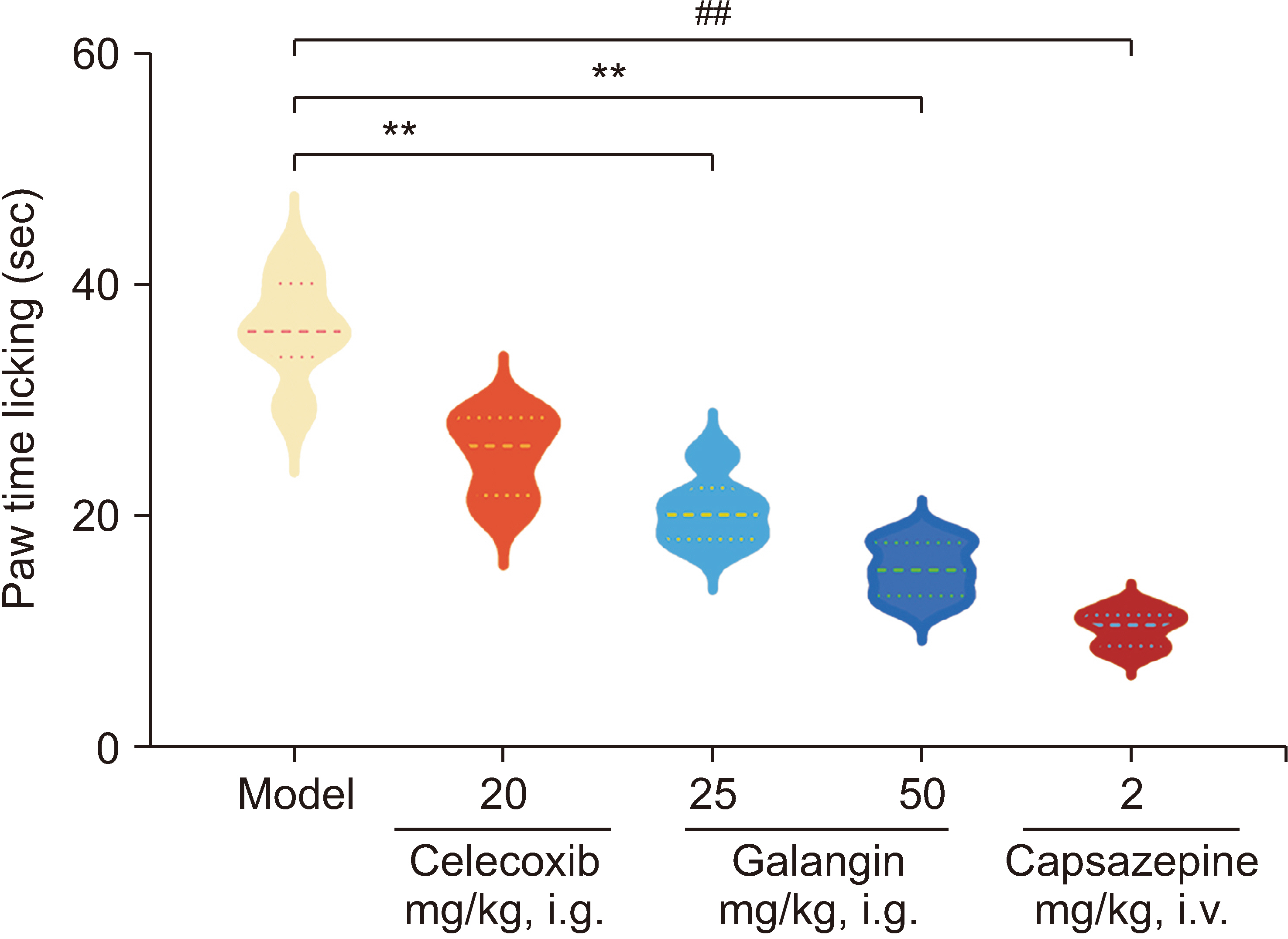
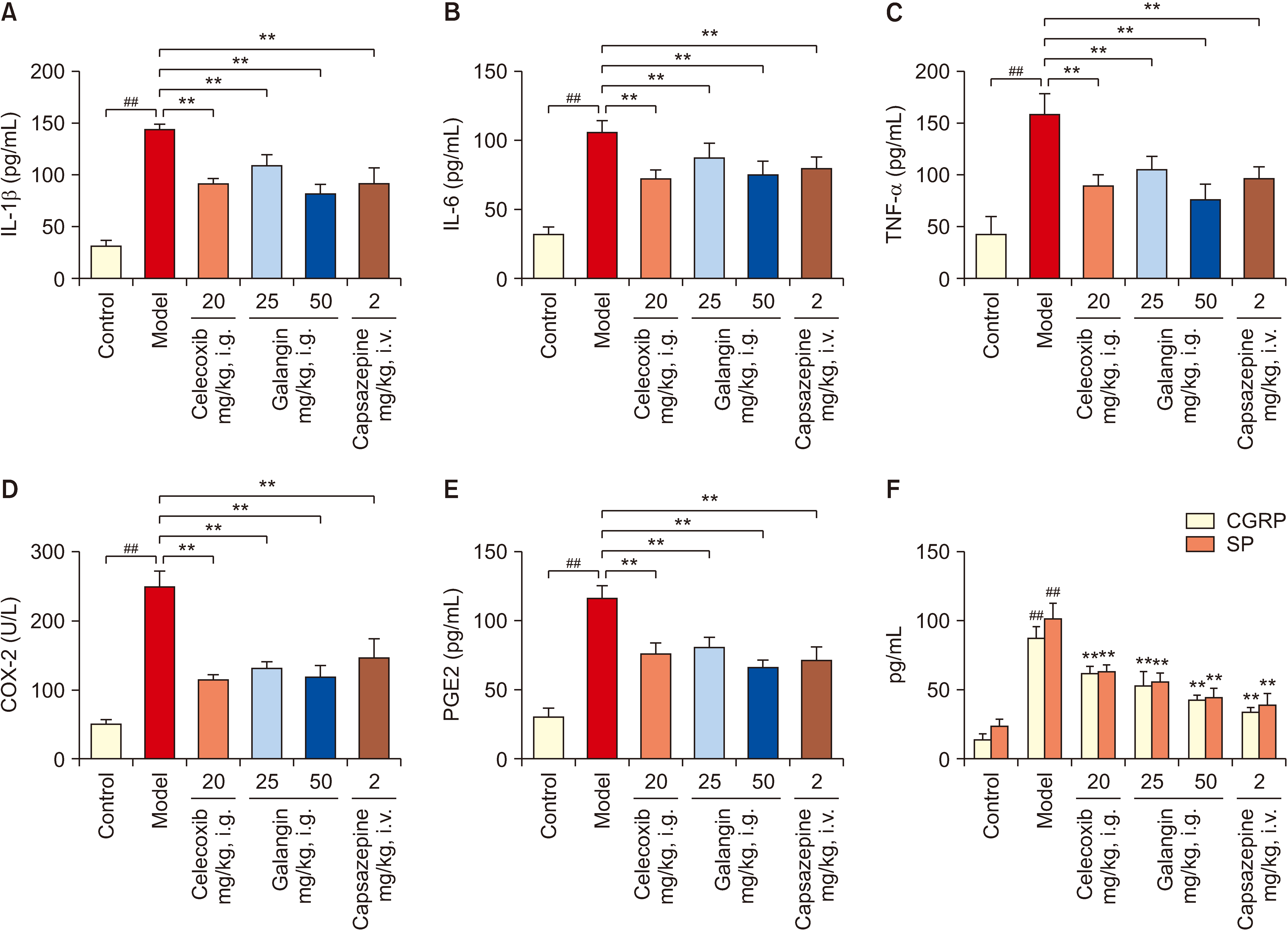
Mice were randomly divided into 4 groups for 7 days: a normal control group, a galangin-treated (25 and 50 mg/kg), and a positive control celecoxib (20 mg/kg). Analgesic and anti-inflammatory effects were evaluated using a hot plate test, acetic acid-induced writhing test, acetic acid-induced vascular permeability test, formalininduced paw licking test, and carrageenan-induced paw swelling test. The interplay between galangin, transient receptor potential vanilloid 1 (TRPV1), NF-κB, COX-2, and TNF-α proteins was evaluated via molecular docking. COX- 2, PGE2, IL-1β, IL-6, and TNF-α levels in serum were measured using ELISA after capsaicin administration (200 nmol/L). TRPV1 expression in the dorsal root ganglion was analyzed by Western blot. The quantities of substance P (SP) and calcitonin gene-related peptide (CGRP) were assessed using qPCR.
Results
Galangin reduced hot plate-induced licking latency, acetic acid-induced contortions, carrageenantriggered foot inflammation, and capillary permeability in mice. It exhibited favorable affinity towards TRPV1, NF- κB, COX-2, and TNF-α, resulting in decreased levels of COX-2, PGE2, IL-1β, IL-6, and TNF-α in serum following capsaicin stimulation. Galangin effectively suppressed the upregulation of TRPV1 protein and associated receptor neuropeptides CGRP and SP mRNA, while concurrently inhibiting the expression of NF-κB, TNF-α, COX-2, and PGE2 mRNA.
Conclusions
Galangin exerts its anti-inflammatory pain effects by inhibiting TRPV1 activation and regulating COX-2, NF-κB/TNF-α expression, providing evidence for the use of galangin in the management of inflammatory pain.
Keyword
Figure
Cited by 1 articles
-
Data sharing and research integrity: lessons from a recent retraction
Francis Sahngun Nahm
Korean J Pain. 2025;38(1):1-3. doi: 10.3344/kjp.23220r.
Reference
-
1. Malin SA, Molliver DC, Koerber HR, Cornuet P, Frye R, Albers KM, et al. 2006; Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci. 26:8588–99. DOI: 10.1523/JNEUROSCI.1726-06.2006. PMID: 16914685. PMCID: PMC6674355.2. Undem BJ, Taylor-Clark T. 2014; Mechanisms underlying the neuronal-based symptoms of allergy. J Allergy Clin Immunol. 133:1521–34. DOI: 10.1016/j.jaci.2013.11.027. PMID: 24433703. PMCID: PMC6483074.
Article3. Julius D. 2013; TRP channels and pain. Annu Rev Cell Dev Biol. 29:355–84. DOI: 10.1146/annurev-cellbio-101011-155833. PMID: 24099085.
Article4. Manion J, Waller MA, Clark T, Massingham JN, Neely GG. 2019; Developing modern pain therapies. Front Neurosci. 13:1370. DOI: 10.3389/fnins.2019.01370. PMID: 31920521. PMCID: PMC6933609.
Article5. Ellis A, Bennett DL. 2013; Neuroinflammation and the generation of neuropathic pain. Br J Anaesth. 111:26–37. DOI: 10.1093/bja/aet128. PMID: 23794642.
Article6. Shen P, Huang Y, Ba X, Lin W, Qin K, Wang H, et al. 2021; Si Miao San attenuates inflammation and oxidative stress in rats with CIA via the modulation of the Nrf2/ARE/PTEN pathway. Evid Based Complement Alternat Med. 2021:2843623. DOI: 10.1155/2021/2843623. PMID: 33628297. PMCID: PMC7892228.
Article7. Che DN, Cho BO, Shin JY, Kang HJ, Kim JS, Oh H, et al. 2019; Apigenin inhibits IL-31 cytokine in human mast cell and mouse skin tissues. Molecules. 24:1290. DOI: 10.3390/molecules24071290. PMID: 30987029. PMCID: PMC6479805.
Article8. Lin K, Deng T, Qu H, Ou H, Huang Q, Gao B, et al. 2023; Gastric protective effect of Alpinia officinarum flavonoids: mediating TLR4/NF-κB and TRPV1 signalling pathways and gastric mucosal healing. Pharm Biol. 61:50–60. DOI: 10.1080/13880209.2022.2152058. PMID: 36541204. PMCID: PMC9788718.
Article9. Abubakar IB, Malami I, Yahaya Y, Sule SM. 2018; A review on the ethnomedicinal uses, phytochemistry and pharmacology of Alpinia officinarum Hance. J Ethnopharmacol. 224:45–62. DOI: 10.1016/j.jep.2018.05.027. PMID: 29803568.
Article10. Chen X, Li X, Zhang X, You L, Cheung PC, Huang R, et al. 2019; Antihyperglycemic and antihyperlipidemic activities of a polysaccharide from Physalis pubescens L. in streptozotocin (STZ)-induced diabetic mice. Food Funct. 10:4868–76. DOI: 10.1039/C9FO00687G. PMID: 31334540.11. Cordenonsi LM, Sponchiado RM, Campanharo SC, Garcia CV, Raffin RP, Schapoval EES. 2017; Study of flavonoids present in pomelo (Citrus maxima) by DSC, UV-VIS, IR, 1H and 13C NMR and MS. Drug Anal Res. 1:31–7. DOI: 10.22456/2527-2616.74097.
Article12. Zhang R, Lu J, Pei G, Huang S. 2023; Galangin rescues Alzheimer's amyloid-β induced mitophagy and brain organoid growth impairment. Int J Mol Sci. 24:3398. DOI: 10.3390/ijms24043398. PMID: 36834819. PMCID: PMC9960784.
Article13. Yang T, Liu H, Yang C, Mo H, Wang X, Song X, et al. 2023; Galangin attenuates myocardial ischemic reperfusion-induced ferroptosis by targeting Nrf2/Gpx4 signaling pathway. Drug Des Devel Ther. 17:2495–511. DOI: 10.2147/DDDT.S409232. PMID: 37637264. PMCID: PMC10460190.
Article14. Zhang F, Yan Y, Zhang LM, Li DX, Li L, Lian WW, et al. 2023; Pharmacological activities and therapeutic potential of galangin, a promising natural flavone, in age-related diseases. Phytomedicine. 120:155061. DOI: 10.1016/j.phymed.2023.155061. PMID: 37689035.
Article15. Hassanein EHM, Abd El-Maksoud MS, Ibrahim IM, Abd-Alhameed EK, Althagafy HS, Mohamed NM, et al. 2023; The molecular mechanisms underlying anti-inflammatory effects of galangin in different diseases. Phytother Res. 37:3161–81. DOI: 10.1002/ptr.7874. PMID: 37246827.
Article16. Thapa R, Afzal O, Alfawaz Altamimi AS, Goyal A, Almalki WH, Alzarea SI, et al. 2023; Galangin as an inflammatory response modulator: an updated overview and therapeutic potential. Chem Biol Interact. 378:110482. DOI: 10.1016/j.cbi.2023.110482. PMID: 37044286.
Article17. Liang X, Wang P, Yang C, Huang F, Wu H, Shi H, et al. 2021; Galangin inhibits gastric cancer growth through enhancing STAT3 mediated ROS production. Front Pharmacol. 12:646628. DOI: 10.3389/fphar.2021.646628. PMID: 33981228. PMCID: PMC8109028.
Article18. Chen K, Xue R, Geng Y, Zhang S. 2022; Galangin inhibited ferroptosis through activation of the PI3K/AKT pathway in vitro and in vivo. FASEB J. 36:e22569. DOI: 10.1096/fj.202200935R. PMID: 36183339.
Article19. Yang L, Ma XY, Mu KX, Dai Y, Xia YF, Wei ZF. 2023; Galangin targets HSP90β to alleviate ulcerative colitis by controlling fatty acid synthesis and subsequent NLRP3 inflammasome activation. Mol Nutr Food Res. 67:e2200755. DOI: 10.1002/mnfr.202200755. PMID: 37002873.
Article20. Chen QX, Zhou L, Long T, Qin DL, Wang YL, Ye Y, et al. 2022; Galangin exhibits neuroprotective effects in 6-OHDA-induced models of Parkinson's disease via the Nrf2/Keap1 pathway. Pharmaceuticals (Basel). 15:1014. DOI: 10.3390/ph15081014. PMID: 36015161. PMCID: PMC9413091.
Article21. Yimer T, Birru EM, Adugna M, Geta M, Emiru YK. 2020; Evaluation of analgesic and anti-inflammatory activities of 80% methanol root extract of Echinops kebericho M. (Asteraceae). J Inflamm Res. 13:647–58. DOI: 10.2147/JIR.S267154. PMID: 33061529. PMCID: PMC7533268.22. Hmamou A, El-Assri EM, El Khomsi M, Kara M, Zuhair Alshawwa S, Al Kamaly O, et al. 2023; Papaver rhoeas L. stem and flower extracts: anti-struvite, anti-inflammatory, analgesic, and antidepressant activities. Saudi Pharm J. 31:101686. DOI: 10.1016/j.jsps.2023.06.019. PMID: 37448842. PMCID: PMC10336831.23. Naiemur Rahman M, Shahin Ahmed K, Ahmed S, Hossain H, Shahid Ud Daula A. 2023; Integrating in vivo and in silico approaches to investigate the potential of Zingiber roseum rhizome extract against pyrexia, inflammation and pain. Saudi J Biol Sci. 30:103624. DOI: 10.1016/j.sjbs.2023.103624. PMID: 36970254. PMCID: PMC10036801.24. Arbain D, Sinaga LMR, Taher M, Susanti D, Zakaria ZA, Khotib J. 2022; Traditional uses, phytochemistry and biological activities of Alocasia species: a systematic review. Front Pharmacol. 13:849704. DOI: 10.3389/fphar.2022.849704. PMID: 35685633. PMCID: PMC9170998.
Article25. Kim HY, Lee HJ, Zuo G, Hwang SH, Park JS, Hong JS, et al. 2022; Antinociceptive activity of the Caesalpinia eriostachys Benth. ethanolic extract, fractions, and isolated compounds in mice. Food Sci Nutr. 10:2381–9. DOI: 10.1002/fsn3.2846. PMID: 35844922. PMCID: PMC9281943.26. Lopes AJO, Vasconcelos CC, Garcia JBS, Dória Pinheiro MS, Pereira FAN, Camelo DS, et al. 2020; Anti-inflammatory and antioxidant activity of pollen extract collected by Scaptotrigona affinis postica: in silico, in vitro, and in vivo studies. Antioxidants (Basel). 9:103. DOI: 10.3390/antiox9020103. PMID: 31991696. PMCID: PMC7070678.
Article27. Hajhashemi V, Sadeghi H, Karimi Madab F. 2024; Anti-inflammatory and antinociceptive effects of sitagliptin in animal models and possible mechanisms involved in the antinociceptive activity. Korean J Pain. 37:26–33. DOI: 10.3344/kjp.23262. PMID: 38123184. PMCID: PMC10764209.
Article28. Azamatov AA, Zhurakulov SN, Vinogradova VI, Tursunkhodzhaeva F, Khinkar RM, Malatani RT, et al. 2023; Evaluation of the local anesthetic activity, acute toxicity, and structure-toxicity relationship in series of synthesized 1-aryltetrahydroisoquinoline alkaloid derivatives in vivo and in silico. Molecules. 28:477. DOI: 10.3390/molecules28020477. PMID: 36677539. PMCID: PMC9864514.
Article29. Yonghak P, Miyata S, Kurganov E. 2020; TRPV1 is crucial for thermal homeostasis in the mouse by heat loss behaviors under warm ambient temperature. Sci Rep. 10:8799. DOI: 10.1038/s41598-020-65703-9. PMID: 32472067. PMCID: PMC7260197.
Article30. Chen C, Yu Z, Lin D, Wang X, Zhang X, Ji F, et al. 2021; Manual acupuncture at ST37 modulates TRPV1 in rats with acute visceral hyperalgesia via phosphatidylinositol 3-kinase/Akt pathway. Evid Based Complement Alternat Med. 2021:5561999. DOI: 10.1155/2021/5561999. PMID: 34646326. PMCID: PMC8505093.
Article31. Luo J, Walters ET, Carlton SM, Hu H. 2013; Targeting pain-evoking transient receptor potential channels for the treatment of pain. Curr Neuropharmacol. 11:652–63. DOI: 10.2174/1570159X113119990040. PMID: 24396340. PMCID: PMC3849790.
Article32. Dou X, Chen R, Yang J, Dai M, Long J, Sun S, et al. 2023; The potential role of T-cell metabolism-related molecules in chronic neuropathic pain after nerve injury: a narrative review. Front Immunol. 14:1107298. DOI: 10.3389/fimmu.2023.1107298. PMID: 37266437. PMCID: PMC10229812.
Article33. Kang MS, Hyun KY. 2020; Antinociceptive and anti-inflammatory effects of Nypa fruticans Wurmb by suppressing TRPV1 in the sciatic neuropathies. Nutrients. 12:135. DOI: 10.3390/nu12010135. PMID: 31947713. PMCID: PMC7019541.
Article34. Rahman MM, Jo HJ, Park CK, Kim YH. 2022; Diosgenin exerts analgesic effects by antagonizing the selective inhibition of transient receptor potential vanilloid 1 in a mouse model of neuropathic pain. Int J Mol Sci. 23:15854. DOI: 10.3390/ijms232415854. PMID: 36555495. PMCID: PMC9784430.
Article35. Jaffal SM, Al-Najjar BO, Abbas MA. 2021; Ononis spinosa alleviated capsaicin-induced mechanical allodynia in a rat model through transient receptor potential vanilloid 1 modulation. Korean J Pain. 34:262–70. DOI: 10.3344/kjp.2021.34.3.262. PMID: 34193633. PMCID: PMC8255156.
Article36. Qnais EY, Alqudah A, Wedyan M, Athamneh RY, Abudalo R, Oqal M, et al. 2023; The analgesic properties of the flavonoid galangal in experimental animal models of nociception. FARMACIA. 71:1054–63. DOI: 10.31925/farmacia.2023.5.20.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of vesicular glutamate transporter in transient receptor potential vanilloid 1-positive neurons in the rat trigeminal ganglion
- The Role of Transient Receptor Potential Channel in Pain
- Transient Receptor Potential Vanilloid Type-1 Channel in Cardiometabolic Protection
- Targeting nerve growth factor for pain relief: pros and cons
- Changes in transient receptor potential vanilloid 1 and transient receptor potential vanilloid 4 in patients with lower urinary tract dysfunction