J Korean Neurosurg Soc.
2024 May;67(3):333-344. 10.3340/jkns.2023.0154.
Prospero Homeobox 1 and Doublecortin Correlate with Neural Damage after Ischemic Stroke
- Affiliations
-
- 1Department of Neurosurgery, Soonchunhyang University Cheonan Hospital, College of Medicine, Soonchunhyang University, Cheonan, Korea
- 2Department of Medical Life Sciences, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Department of Neurosurgery, Uijeongbu St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2554871
- DOI: http://doi.org/10.3340/jkns.2023.0154
Abstract
Objective
: Markers of neuroinflammation during ischemic stroke are well characterized, but additional markers of neural damage are lacking. The study identified associations of behavioral disorders after stroke with histologic neural damage and molecular biological change.
Methods
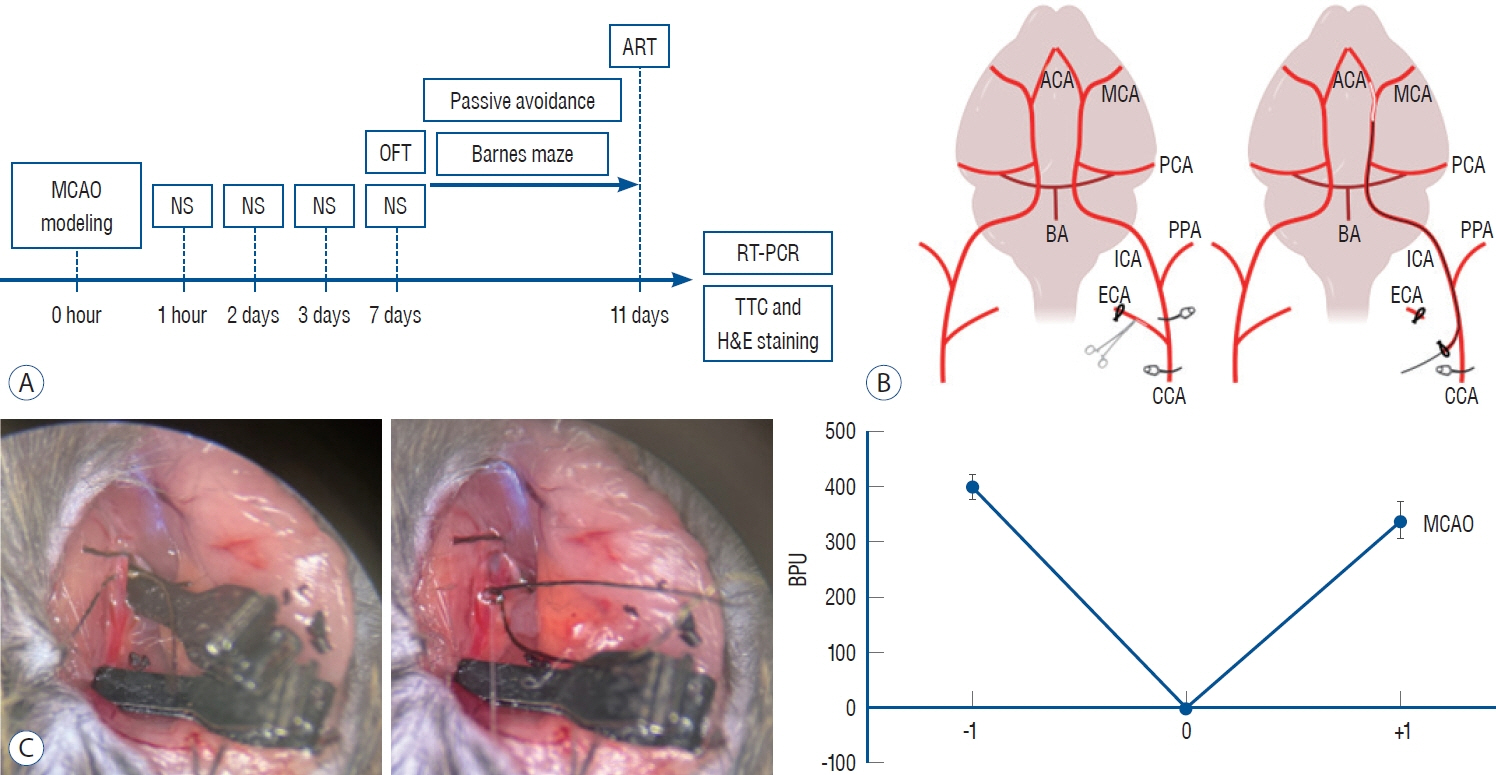
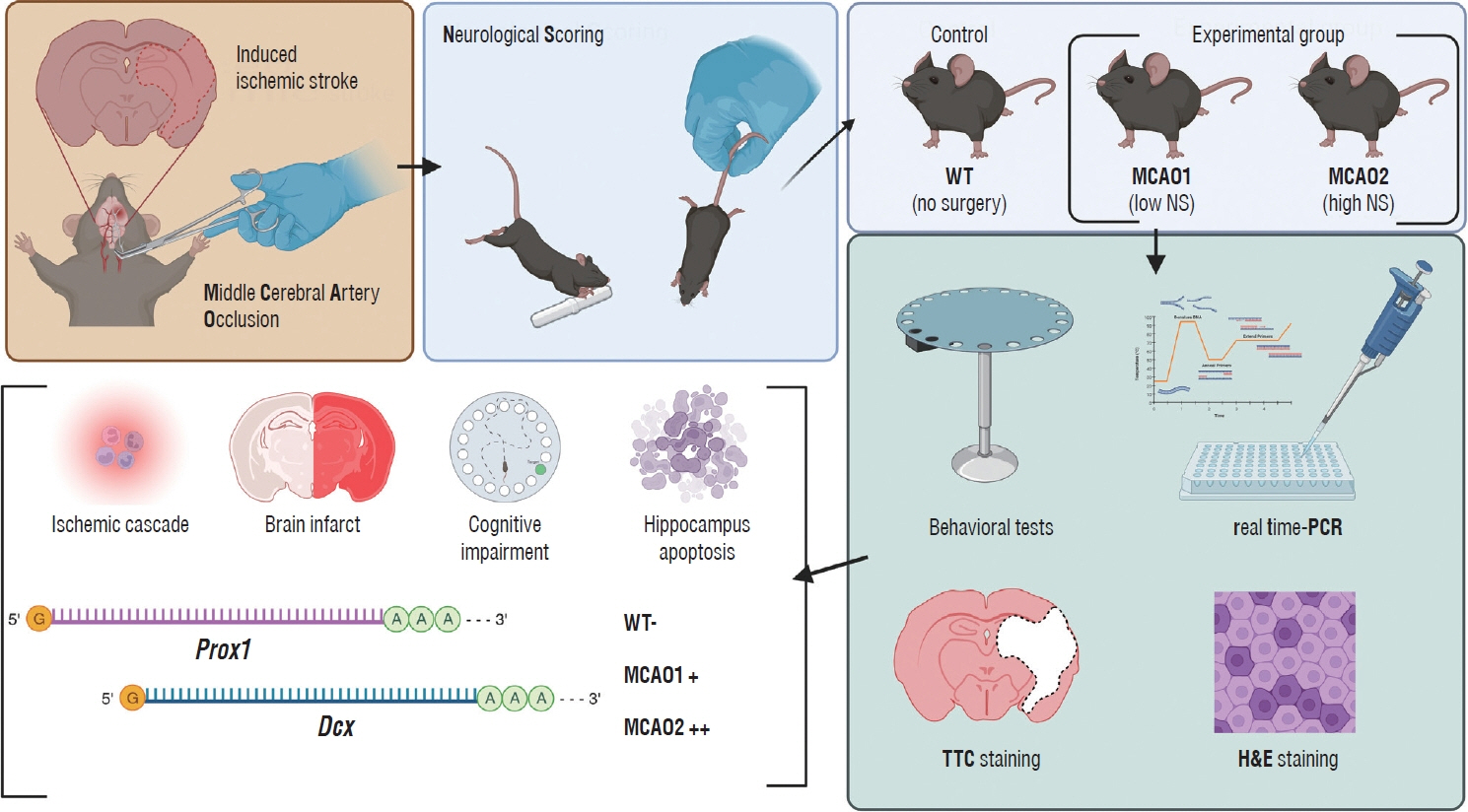
: Eight-week-old, 25 g male mice of the C57BL/6J strain were subjected to middle cerebral artery occlusion (MCAO) to induce ischemic stroke. The control group was a healthy wild type (WT), and the experimental group were designed as a low severity MCAO1 and a high severity MCAO2 based on post-stroke neurological scoring. All groups underwent behavioral tests, realtime polymerase chain reaction, triphenyltetrazolium chloride (TTC) staining and Hematoxylin and Eosin staining. One-way analysis of variance was used to analyze statistical significance between groups.
Results
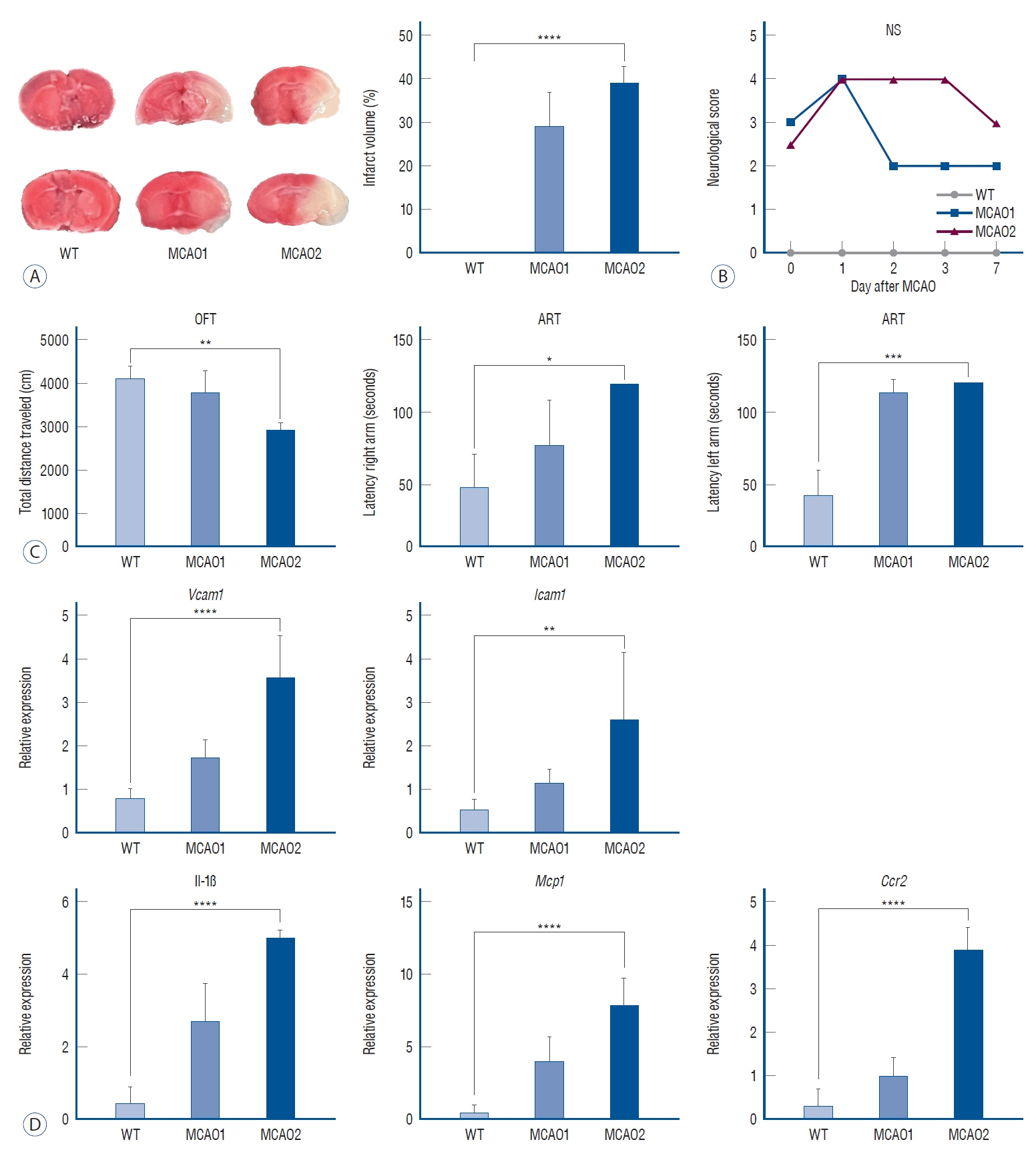
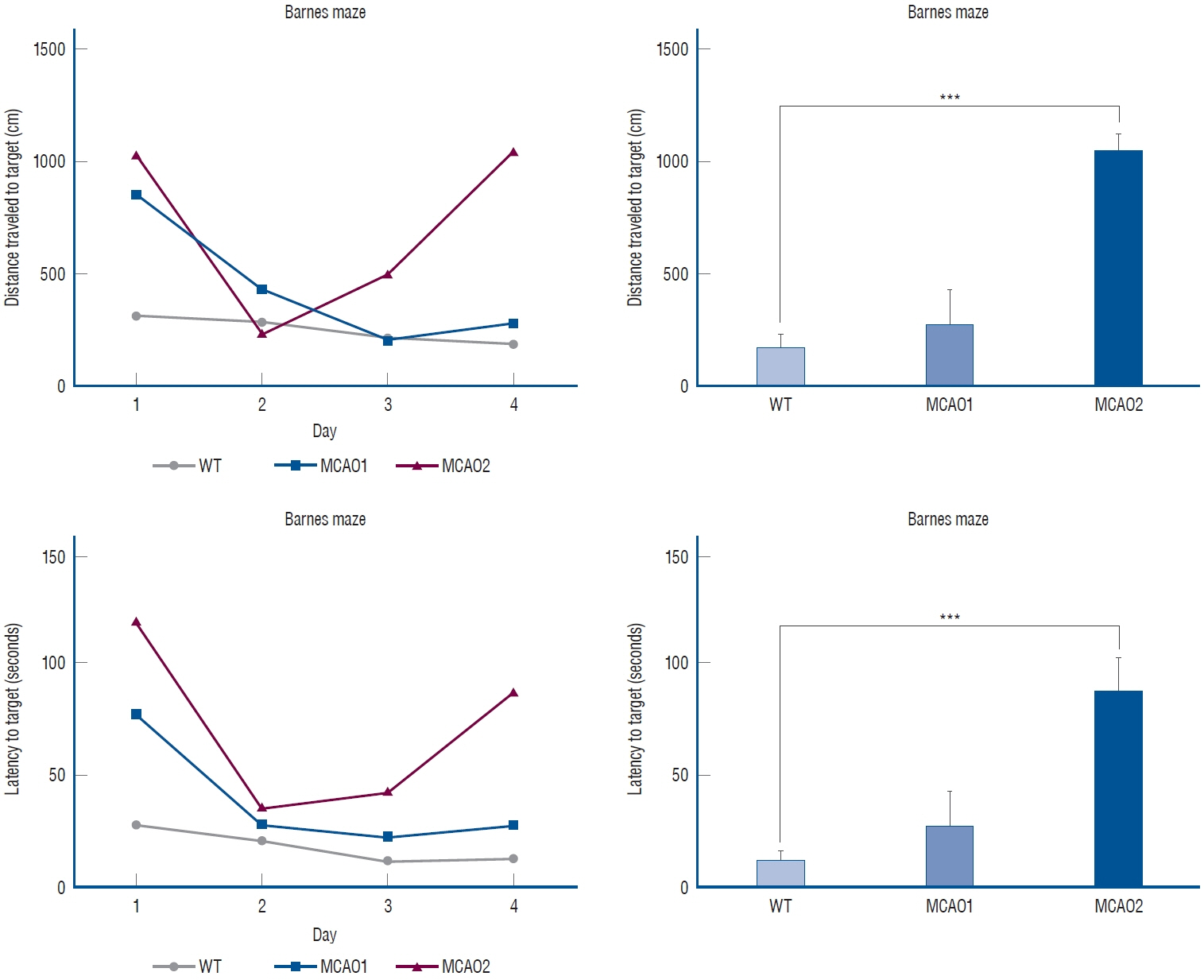
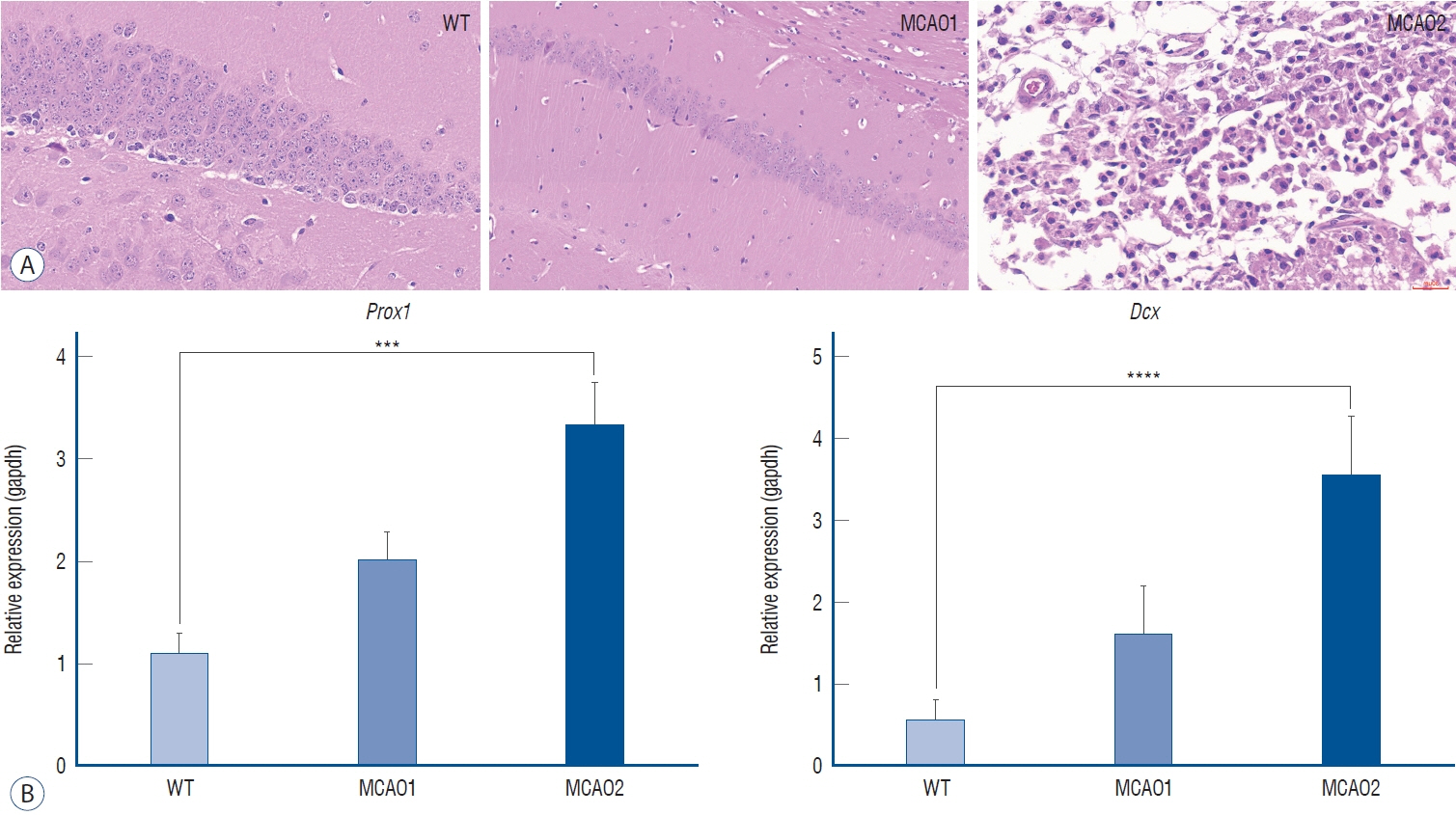
: In TTC staining, MCAO1 showed 29.02% and MCAO2 showed 38.94% infarct volume (p<0.0001). The pro-inflammatory cytokine interleukin (IL)-1β was most highly expressed in MCAO2 (WT 0.44 vs. MCAO1 2.69 vs. MCAO2 5.02, p<0.0001). From the distance to target in the Barnes maze test, WT had a distance of 178 cm, MCAO1 had a distance of 276 cm, and MCAO2 had a distance of 1051 (p=0.0015). The latency to target was 13.3 seconds for WT, 27.9 seconds for MCAO1, and 87.9 seconds for MCAO2 (p=0.0007). Prospero homeobox 1 (Prox1) was most highly expressed in MCAO2 (p=0.0004). Doublecortin (Dcx) was most highly expressed in MCAO2 (p<0.0001).
Conclusion
: The study demonstrated that histological damage to neural cells and changes in brain mRNA expression were associated with behavioral impairment after ischemic stroke. Prox1 and Dcx may be biomarkers of neural damage associated with long-term cognitive decline, and increased expression at the mRNA level was consistent with neural damage and long-term cognitive dysfunction.
Figure
Reference
-
References
1. Aggleton JP, Pralus A, Nelson AJ, Hornberger M. Thalamic pathology and memory loss in early Alzheimer’s disease: moving the focus from the medial temporal lobe to Papez circuit. Brain. 139(Pt 7):1877–1890. 2016.2. Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 467:1–10. 2003.
Article3. Bustamante A, Simats A, Vilar-Bergua A, García-Berrocoso T, Montaner J. Blood/brain biomarkers of inflammation after stroke and their association with outcome: from C-reactive protein to damage-associated molecular patterns. Neurotherapeutics. 13:671–684. 2016.4. Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 91:3652–3656. 1994.5. Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. 63:272–287. 2008.
Article6. Deb P, Sharma S, Hassan KM. Pathophysiologic mechanisms of acute ischemic stroke: an overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology. 17:197–218. 2010.
Article7. Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 1:41–50. 2000.
Article8. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 387:1723–1731. 2016.9. Huo Y, Ley K. Adhesion molecules and atherogenesis. Acta Physiol Scand. 173:35–43. 2001.
Article10. Iwano T, Masuda A, Kiyonari H, Enomoto H, Matsuzaki F. Prox1 postmitotically defines dentate gyrus cells by specifying granule cell identity over CA3 pyramidal cell fate in the hippocampus. Development. 139:3051–3062. 2012.11. Jokinen H, Melkas S, Ylikoski R, Pohjasvaara T, Kaste M, Erkinjuntti T, et al. Post-stroke cognitive impairment is common even after successful clinical recovery. Eur J Neurol. 22:1288–1294. 2015.
Article12. Karalay O, Doberauer K, Vadodaria KC, Knobloch M, Berti L, Miquelajauregui A, et al. Prospero-related homeobox 1 gene (Prox1) is regulated by canonical Wnt signaling and has a stage-specific role in adult hippocampal neurogenesis. Proc Natl Acad Sci U S A. 108:5807–5812. 2011.13. Koton S, Pike JR, Johansen M, Knopman DS, Lakshminarayan K, Mosley T, et al. Association of ischemic stroke incidence, severity, and recurrence with dementia in the atherosclerosis risk in communities cohort study. JAMA Neurol. 79:271–280. 2022.
Article14. Lackland DT, Roccella EJ, Deutsch AF, Fornage M, George MG, Howard G, et al. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke. 45:315–353. 2014.
Article15. Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 8:741–754. 2009.
Article16. Lavado A, Lagutin OV, Chow LM, Baker SJ, Oliver G. Prox1 is required for granule cell maturation and intermediate progenitor maintenance during brain neurogenesis. PLoS Biol. 8:e1000460. 2010.17. Liu T, McDonnell PC, Young PR, White RF, Siren AL, Hallenbeck JM, et al. Interleukin-1 beta mRNA expression in ischemic rat cortex. Stroke. 24:1746–1750. discussion 1750-1751. 1993.18. Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 4:399–415. 2003.19. Lo JW, Crawford JD, Desmond DW, Godefroy O, Jokinen H, Mahinrad S, et al. Profile of and risk factors for poststroke cognitive impairment in diverse ethnoregional groups. Neurology. 93:e2257–e2271. 2019.20. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 20:84–91. 1989.
Article21. Lv ZM, Zhao RJ, Zhi XS, Huang Y, Chen JY, Song NN, et al. Expression of DCX and transcription factor profiling in photothrombosis-induced focal ischemia in mice. Front Cell Neurosci. 12:455. 2018.22. Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 36:587–597. 2013.
Article23. Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 380:2197–2223. 2012.24. Nawabi H, Belin S, Cartoni R, Williams PR, Wang C, Latremolière A, et al. Doublecortin-like kinases promote neuronal survival and induce growth cone reformation via distinct mechanisms. Neuron. 88:704–719. 2015.
Article25. Olmez I, Ozyurt H. Reactive oxygen species and ischemic cerebrovascular disease. Neurochem Int. 60:208–212. 2012.
Article26. Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 8:1006–1018. 2009.27. Pendlebury ST, Rothwell PM; Oxford Vascular Study. Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: analysis of the population-based Oxford Vascular Study. Lancet Neurol. 18:248–258. 2019.28. Rost NS, Brodtmann A, Pase MP, van Veluw SJ, Biffi A, Duering M, et al. Post-stroke cognitive impairment and dementia. Circ Res. 130:1252–1271. 2022.
Article29. Toyoda K, Yoshimura S, Nakai M, Koga M, Sasahara Y, Sonoda K, et al. Twenty-year change in severity and outcome of ischemic and hemorrhagic strokes. JAMA Neurol. 79:61–69. 2022.
Article30. Ullman MT. Contributions of memory circuits to language: the declarative/procedural model. Cognition. 92:231–270. 2004.
Article31. Zarei M, Patenaude B, Damoiseaux J, Morgese C, Smith S, Matthews PM, et al. Combining shape and connectivity analysis: an MRI study of thalamic degeneration in Alzheimer’s disease. Neuroimage. 49:1–8. 2010.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neural Stem Cells and Ischemic Brain
- Current Status and Therapeutic Perspectives for the Stem Cells Treatment of Ischemic Stroke
- Antiplatelet Therapy for Secondary Stroke Prevention in Patients with Ischemic Stroke or Transient Ischemic Attack
- Mesenchymal stem cells promote proliferation of endogenous neural stem cells and survival of newborn cells in a rat stroke model
- Collateral Circulation in Ischemic Stroke: An Updated Review