Diabetes Metab J.
2024 Jan;48(1):37-52. 10.4093/dmj.2023.0193.
Attention to Innate Circadian Rhythm and the Impact of Its Disruption on Diabetes
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea
- 2BK21 FOUR R&E Center for Learning Health Systems, Korea University, Seoul, Korea
- KMID: 2551260
- DOI: http://doi.org/10.4093/dmj.2023.0193
Abstract
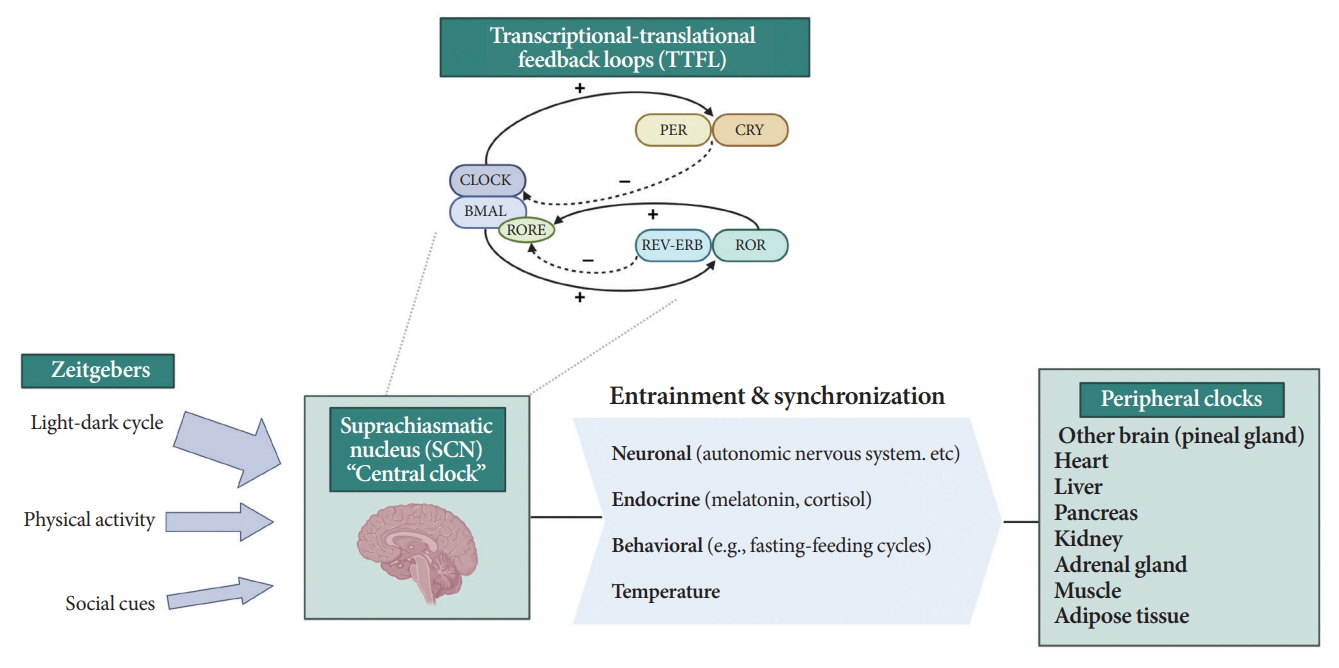
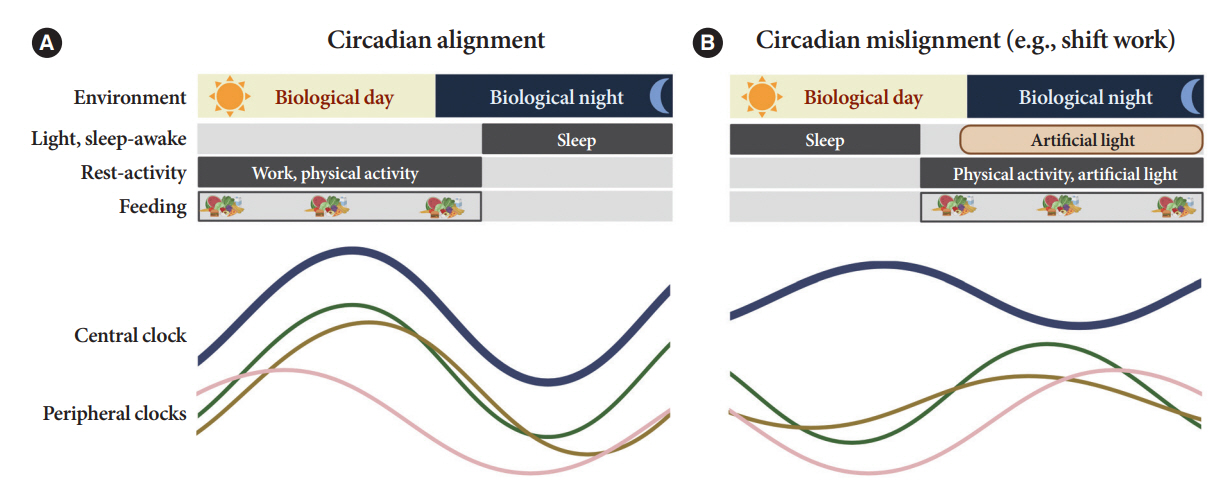
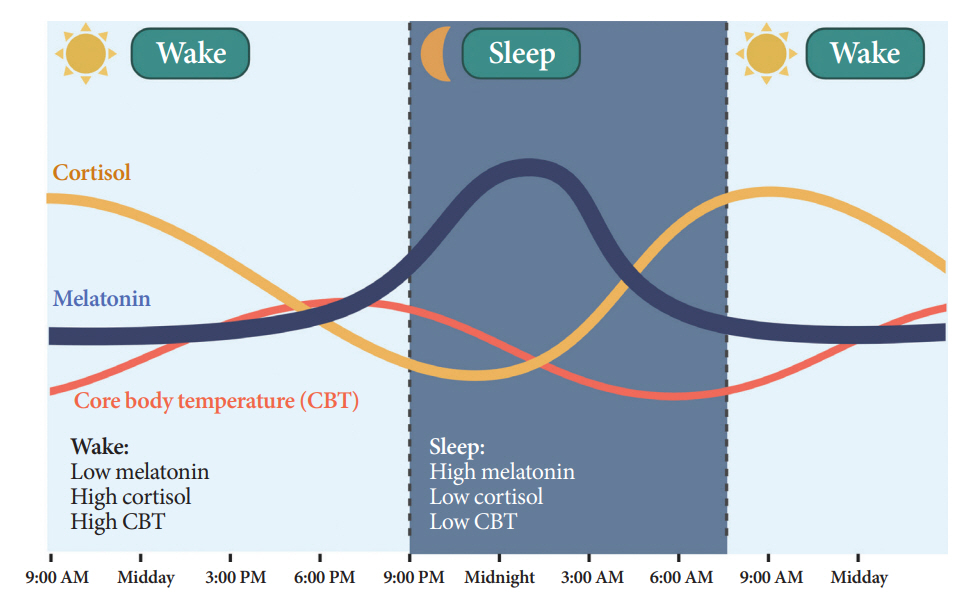
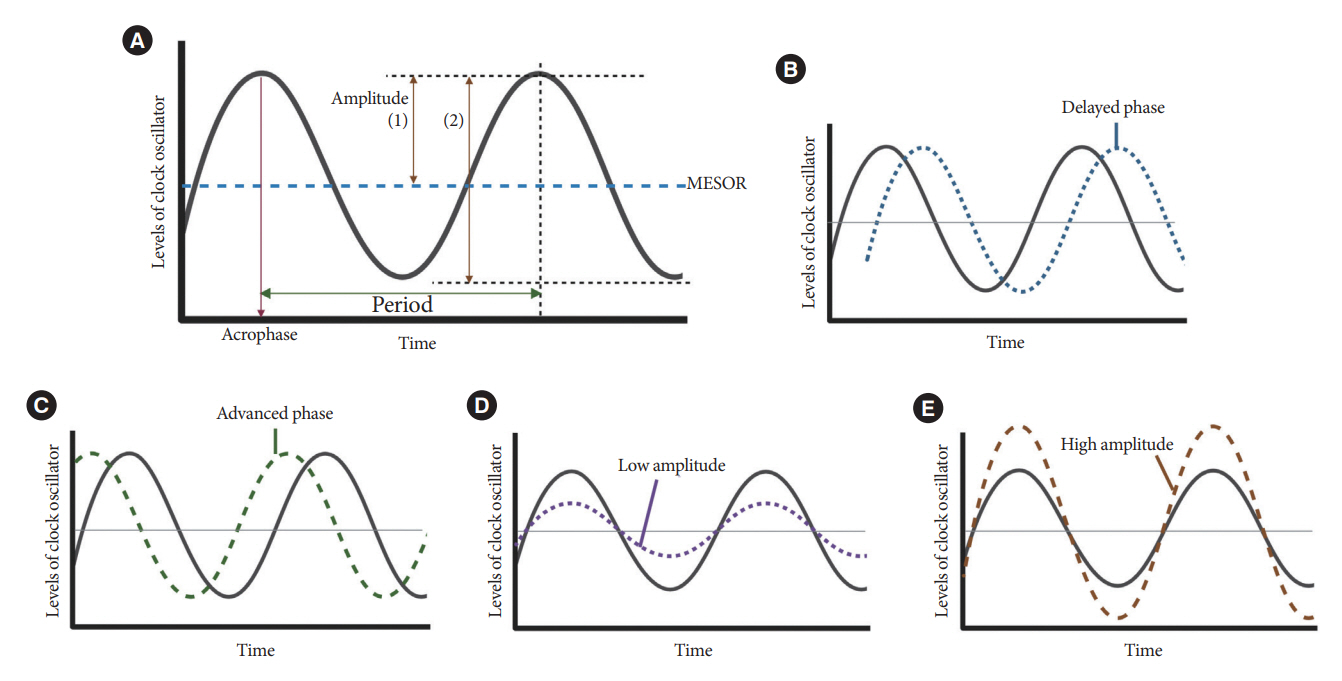
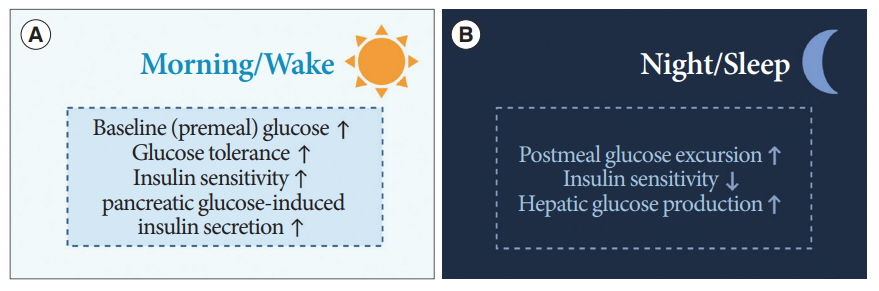
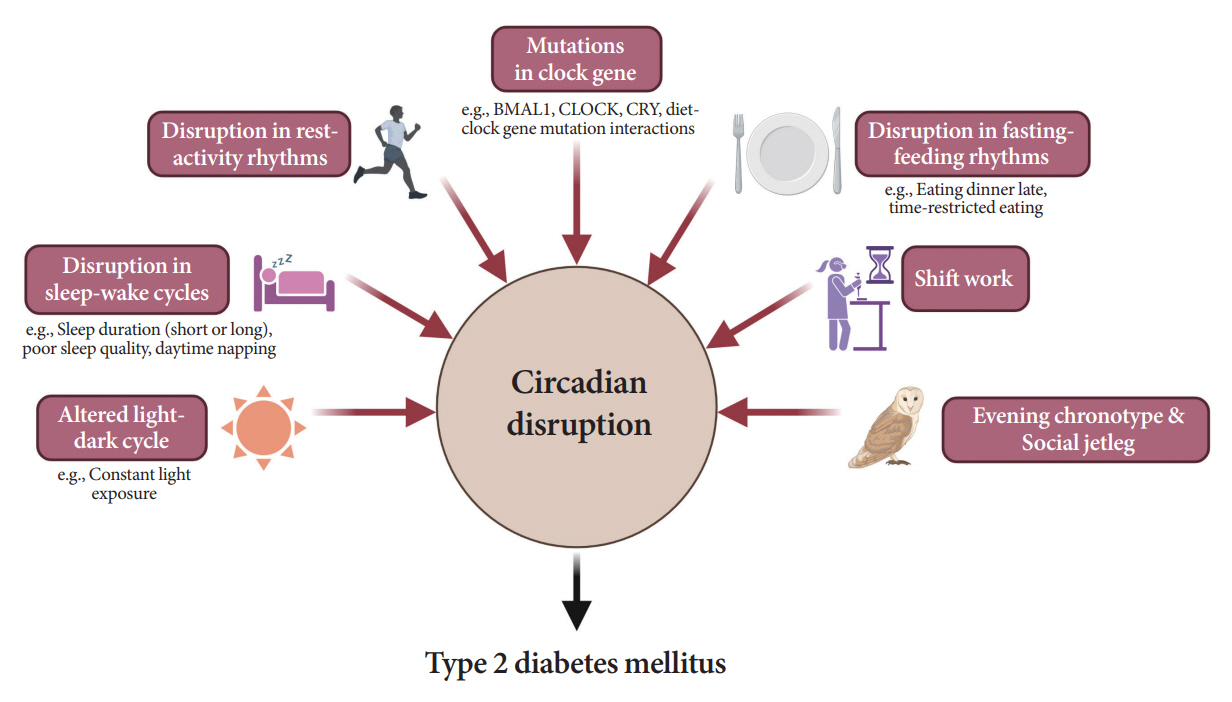
- Novel strategies are required to reduce the risk of developing diabetes and/or clinical outcomes and complications of diabetes. In this regard, the role of the circadian system may be a potential candidate for the prevention of diabetes. We reviewed evidence from animal, clinical, and epidemiological studies linking the circadian system to various aspects of the pathophysiology and clinical outcomes of diabetes. The circadian clock governs genetic, metabolic, hormonal, and behavioral signals in anticipation of cyclic 24-hour events through interactions between a “central clock” in the suprachiasmatic nucleus and “peripheral clocks” in the whole body. Currently, circadian rhythmicity in humans can be subjectively or objectively assessed by measuring melatonin and glucocorticoid levels, core body temperature, peripheral blood, oral mucosa, hair follicles, rest-activity cycles, sleep diaries, and circadian chronotypes. In this review, we summarized various circadian misalignments, such as altered light-dark, sleep-wake, rest-activity, fasting-feeding, shift work, evening chronotype, and social jetlag, as well as mutations in clock genes that could contribute to the development of diabetes and poor glycemic status in patients with diabetes. Targeting critical components of the circadian system could deliver potential candidates for the treatment and prevention of type 2 diabetes mellitus in the future.
Keyword
Figure
Cited by 1 articles
-
Artificial Light at Night and Type 2 Diabetes Mellitus
Jong-Ha Baek, Yong Zhu, Chandra L. Jackson, Yong-Moon Mark Park
Diabetes Metab J. 2024;48(5):847-863. doi: 10.4093/dmj.2024.0237.
Reference
-
1. Defronzo RA. Banting lecture: from the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009; 58:773–95.2. Zimmet P, Alberti KG, Magliano DJ, Bennett PH. Diabetes mellitus statistics on prevalence and mortality: facts and fallacies. Nat Rev Endocrinol. 2016; 12:616–22.3. Mason IC, Qian J, Adler GK, Scheer FA. Impact of circadian disruption on glucose metabolism: implications for type 2 diabetes. Diabetologia. 2020; 63:462–72.4. Javeed N, Matveyenko AV. Circadian etiology of type 2 diabetes mellitus. Physiology (Bethesda). 2018; 33:138–50.5. Poggiogalle E, Jamshed H, Peterson CM. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism. 2018; 84:11–27.6. Huang W, Ramsey KM, Marcheva B, Bass J. Circadian rhythms, sleep, and metabolism. J Clin Invest. 2011; 121:2133–41.7. Peng X, Fan R, Xie L, Shi X, Dong K, Zhang S, et al. A growing link between circadian rhythms, type 2 diabetes mellitus and Alzheimer’s disease. Int J Mol Sci. 2022; 23:504.8. Schibler U, Sassone-Corsi P. A web of circadian pacemakers. Cell. 2002; 111:919–22.9. Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017; 18:164–79.10. Albrecht U. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron. 2012; 74:246–60.11. Buhr ED, Takahashi JS. Molecular components of the Mammalian circadian clock. Handb Exp Pharmacol. 2013; 217:3–27.12. Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999; 284:2177–81.13. Crnko S, Schutte H, Doevendans PA, Sluijter JP, van Laake LW. Minimally invasive ways of determining circadian rhythms in humans. Physiology (Bethesda). 2021; 36:7–20.14. Hood S, Amir S. Neurodegeneration and the circadian clock. Front Aging Neurosci. 2017; 9:170.15. Peng F, Li X, Xiao F, Zhao R, Sun Z. Circadian clock, diurnal glucose metabolic rhythm, and dawn phenomenon. Trends Neurosci. 2022; 45:471–82.16. Morin LP. Neuroanatomy of the extended circadian rhythm system. Exp Neurol. 2013; 243:4–20.17. Buijs RM. The autonomic nervous system: a balancing act. Handb Clin Neurol. 2013; 117:1–11.18. Flaa A, Aksnes TA, Kjeldsen SE, Eide I, Rostrup M. Increased sympathetic reactivity may predict insulin resistance: an 18-year follow-up study. Metabolism. 2008; 57:1422–7.19. Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999; 354:1435–9.20. Smale L, Lee T, Nunez AA. Mammalian diurnality: some facts and gaps. J Biol Rhythms. 2003; 18:356–66.21. Tordjman S, Chokron S, Delorme R, Charrier A, Bellissant E, Jaafari N, et al. Melatonin: pharmacology, functions and therapeutic benefits. Curr Neuropharmacol. 2017; 15:434–43.22. Pandi-Perumal SR, Smits M, Spence W, Srinivasan V, Cardinali DP, Lowe AD, et al. Dim light melatonin onset (DLMO): a tool for the analysis of circadian phase in human sleep and chronobiological disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2007; 31:1–11.23. Cipolla-Neto J, Amaral FG. Melatonin as a hormone: new physiological and clinical insights. Endocr Rev. 2018; 39:990–1028.24. Burgess HJ, Revell VL, Eastman CI. A three pulse phase response curve to three milligrams of melatonin in humans. J Physiol. 2008; 586:639–47.25. Jin X, von Gall C, Pieschl RL, Gribkoff VK, Stehle JH, Reppert SM, et al. Targeted disruption of the mouse Mel(1b) melatonin receptor. Mol Cell Biol. 2003; 23:1054–60.26. Benloucif S, Burgess HJ, Klerman EB, Lewy AJ, Middleton B, Murphy PJ, et al. Measuring melatonin in humans. J Clin Sleep Med. 2008; 4:66–9.27. Kakihana R, Moore JA. Circadian rhythm of corticosterone in mice: the effect of chronic consumption of alcohol. Psychopharmacologia. 1976; 46:301–5.28. Morris CJ, Aeschbach D, Scheer FA. Circadian system, sleep and endocrinology. Mol Cell Endocrinol. 2012; 349:91–104.29. Kim BH, Joo Y, Kim MS, Choe HK, Tong Q, Kwon O. Effects of intermittent fasting on the circulating levels and circadian rhythms of hormones. Endocrinol Metab (Seoul). 2021; 36:745–56.30. Nicolaides NC, Charmandari E, Chrousos GP, Kino T. Circadian endocrine rhythms: the hypothalamic-pituitary-adrenal axis and its actions. Ann N Y Acad Sci. 2014; 1318:71–80.31. Ulrich-Lai YM, Arnhold MM, Engeland WC. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am J Physiol Regul Integr Comp Physiol. 2006; 290:R1128–35.32. Li MD, Xin H, Yuan Y, Yang X, Li H, Tian D, et al. Circadian clock-controlled checkpoints in the pathogenesis of complex disease. Front Genet. 2021; 12:721231.33. Son GH, Chung S, Choe HK, Kim HD, Baik SM, Lee H, et al. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc Natl Acad Sci U S A. 2008; 105:20970–5.34. Lederbogen F, Hummel J, Fademrecht C, Krumm B, Kuhner C, Deuschle M, et al. Flattened circadian cortisol rhythm in type 2 diabetes. Exp Clin Endocrinol Diabetes. 2011; 119:573–5.35. Zulley J, Wever R, Aschoff J. The dependence of onset and duration of sleep on th circadian rhythm of rectal temperature. Pflugers Arch. 1981; 391:314–8.36. Benloucif S, Guico MJ, Reid KJ, Wolfe LF, L’hermite-Baleriaux M, Zee PC. Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J Biol Rhythms. 2005; 20:178–88.37. Jakubowicz D, Wainstein J, Landau Z, Raz I, Ahren B, Chapnik N, et al. Influences of breakfast on clock gene expression and postprandial glycemia in healthy individuals and individuals with diabetes: a randomized clinical trial. Diabetes Care. 2017; 40:1573–9.38. Yang MY, Lin PW, Lin HC, Lin PM, Chen IY, Friedman M, et al. Alternations of circadian clock genes expression and oscillation in obstructive sleep apnea. J Clin Med. 2019; 8:1634.39. Cajochen C, Jud C, Munch M, Kobialka S, Wirz-Justice A, Albrecht U. Evening exposure to blue light stimulates the expression of the clock gene PER2 in humans. Eur J Neurosci. 2006; 23:1082–6.40. Novakova M, Sladek M, Sumova A. Human chronotype is determined in bodily cells under real-life conditions. Chronobiol Int. 2013; 30:607–17.41. Akashi M, Soma H, Yamamoto T, Tsugitomi A, Yamashita S, Yamamoto T, et al. Noninvasive method for assessing the human circadian clock using hair follicle cells. Proc Natl Acad Sci U S A. 2010; 107:15643–8.42. Ferrante A, Gellerman D, Ay A, Woods KP, Filipowicz AM, Jain K, et al. Diurnal preference predicts phase differences in expression of human peripheral circadian clock genes. J Circadian Rhythms. 2015; 13:4.43. Watanabe M, Hida A, Kitamura S, Enomoto M, Ohsawa Y, Katayose Y, et al. Rhythmic expression of circadian clock genes in human leukocytes and beard hair follicle cells. Biochem Biophys Res Commun. 2012; 425:902–7.44. Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003; 26:342–92.45. Lee JH, Moon E, Park J, Oh CE, Hong YR, Yoon M. Optimization of analysis of circadian rest-activity rhythm using cosinor analysis in mice. Psychiatry Investig. 2022; 19:380–5.46. Cornelissen G. Cosinor-based rhythmometry. Theor Biol Med Model. 2014; 11:16.47. Cho CH, Lee T, Kim MG, In HP, Kim L, Lee HJ. Mood prediction of patients with mood disorders by machine learning using passive digital phenotypes based on the circadian rhythm: prospective observational cohort study. J Med Internet Res. 2019; 21:e11029.48. Reid KJ. Assessment of circadian rhythms. Neurol Clin. 2019; 37:505–26.49. Leng Y, Musiek ES, Hu K, Cappuccio FP, Yaffe K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 2019; 18:307–18.50. Yeung CH, Bauer C, Xiao Q. Associations between actigraphy-derived rest-activity rhythm characteristics and hypertension in United States adults. J Sleep Res. 2023; 32:e13854.51. Lee HA, Lee HJ, Moon JH, Lee T, Kim MG, In H, et al. Comparison of wearable activity tracker with actigraphy for sleep evaluation and circadian rest-activity rhythm measurement in healthy young adults. Psychiatry Investig. 2017; 14:179–85.52. Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014; 146:1387–94.53. Di Milia L, Adan A, Natale V, Randler C. Reviewing the psychometric properties of contemporary circadian typology measures. Chronobiol Int. 2013; 30:1261–71.54. Waterhouse J, Folkard S, Van Dongen H, Minors D, Owens D, Kerkhof G, et al. Temperature profiles, and the effect of sleep on them, in relation to morningness-eveningness in healthy female subjects. Chronobiol Int. 2001; 18:227–47.55. Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006; 23:497–509.56. Malone SK, Patterson F, Lu Y, Lozano A, Hanlon A. Ethnic differences in sleep duration and morning-evening type in a population sample. Chronobiol Int. 2016; 33:10–21.57. Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976; 4:97–110.58. Juda M, Vetter C, Roenneberg T. The Munich ChronoType Questionnaire for Shift-Workers (MCTQShift). J Biol Rhythms. 2013; 28:130–40.59. Parsons MJ, Moffitt TE, Gregory AM, Goldman-Mellor S, Nolan PM, Poulton R, et al. Social jetlag, obesity and metabolic disorder: investigation in a cohort study. Int J Obes (Lond). 2015; 39:842–8.60. Challet E, Malan A, Turek FW, Van Reeth O. Daily variations of blood glucose, acid-base state and PCO2 in rats: effect of light exposure. Neurosci Lett. 2004; 355:131–5.61. Cailotto C, La Fleur SE, Van Heijningen C, Wortel J, Kalsbeek A, Feenstra M, et al. The suprachiasmatic nucleus controls the daily variation of plasma glucose via the autonomic output to the liver: are the clock genes involved? Eur J Neurosci. 2005; 22:2531–40.62. Iwayama K, Onishi T, Maruyama K, Takahashi H. Diurnal variation in the glycogen content of the human liver using 13 C MRS. NMR Biomed. 2020; 33:e4289.63. La Fleur SE. Daily rhythms in glucose metabolism: suprachiasmatic nucleus output to peripheral tissue. J Neuroendocrinol. 2003; 15:315–22.64. Nakagawa H, Okumura N. Coordinated regulation of circadian rhythms and homeostasis by the suprachiasmatic nucleus. Proc Jpn Acad Ser B Phys Biol Sci. 2010; 86:391–409.65. Foppen E, Tan AA, Ackermans MT, Fliers E, Kalsbeek A. Suprachiasmatic nucleus neuropeptides and their control of endogenous glucose production. J Neuroendocrinol. 2016; 28:12365.66. Saad A, Dalla Man C, Nandy DK, Levine JA, Bharucha AE, Rizza RA, et al. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes. 2012; 61:2691–700.67. Takahashi M, Ozaki M, Kang MI, Sasaki H, Fukazawa M, Iwakami T, et al. Effects of meal timing on postprandial glucose metabolism and blood metabolites in healthy adults. Nutrients. 2018; 10:1763.68. Seshadri N, Doucette CA. Circadian regulation of the pancreatic beta cell. Endocrinology. 2021; 162:bqab089.69. Stenvers DJ, Scheer FA, Schrauwen P, la Fleur SE, Kalsbeek A. Circadian clocks and insulin resistance. Nat Rev Endocrinol. 2019; 15:75–89.70. Papazoglou I, Lee JH, Cui Z, Li C, Fulgenzi G, Bahn YJ, et al. A distinct hypothalamus-to-β cell circuit modulates insulin secretion. Cell Metab. 2022; 34:285–98.71. Perelis M, Marcheva B, Ramsey KM, Schipma MJ, Hutchison AL, Taguchi A, et al. Pancreatic β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science. 2015; 350:aac4250.72. Hogenboom R, Kalsbeek MJ, Korpel NL, de Goede P, Koenen M, Buijs RM, et al. Loss of arginine vasopressin- and vasoactive intestinal polypeptide-containing neurons and glial cells in the suprachiasmatic nucleus of individuals with type 2 diabetes. Diabetologia. 2019; 62:2088–93.73. Schmidt MI, Hadji-Georgopoulos A, Rendell M, Margolis S, Kowarski A. The dawn phenomenon, an early morning glucose rise: implications for diabetic intraday blood glucose variation. Diabetes Care. 1981; 4:579–85.74. Monnier L, Colette C, Dejager S, Owens D. Magnitude of the dawn phenomenon and its impact on the overall glucose exposure in type 2 diabetes: is this of concern? Diabetes Care. 2013; 36:4057–62.75. Li C, Ma X, Yin J, Mo Y, Zhang L, Lu J, et al. The dawn phenomenon across the glycemic continuum: implications for defining dysglycemia. Diabetes Res Clin Pract. 2020; 166:108308.76. Radziuk J, Pye S. Diurnal rhythm in endogenous glucose production is a major contributor to fasting hyperglycaemia in type 2 diabetes: suprachiasmatic deficit or limit cycle behaviour? Diabetologia. 2006; 49:1619–28.77. Ding G, Li X, Hou X, Zhou W, Gong Y, Liu F, et al. REV-ERB in GABAergic neurons controls diurnal hepatic insulin sensitivity. Nature. 2021; 592:763–7.78. Paing AC, McMillan KA, Kirk AF, Collier A, Hewitt A, Dunstan D, et al. Diurnal patterns of objectively measured sedentary time and interruptions to sedentary time are associated with glycaemic indices in type 2 diabetes. J Sci Med Sport. 2020; 23:1074–9.79. Zheng X, Qi Y, Bi L, Shi W, Zhang Y, Zhao D, et al. Effects of exercise on blood glucose and glycemic variability in type 2 diabetic patients with Dawn phenomenon. Biomed Res Int. 2020; 2020:6408724.80. Mokhlesi B, Grimaldi D, Beccuti G, Van Cauter E. Effect of one week of CPAP treatment of obstructive sleep apnoea on 24-hour profiles of glucose, insulin and counter-regulatory hormones in type 2 diabetes. Diabetes Obes Metab. 2017; 19:452–6.81. Paing AC, McMillan KA, Kirk AF, Collier A, Hewitt A, Chastin SF. Dose-response between frequency of interruption of sedentary time and fasting glucose, the dawn phenomenon and night-time glucose in type 2 diabetes. Diabet Med. 2019; 36:376–82.82. Jacobi D, Liu S, Burkewitz K, Kory N, Knudsen NH, Alexander RK, et al. Hepatic Bmal1 regulates rhythmic mitochondrial dynamics and promotes metabolic fitness. Cell Metab. 2015; 22:709–20.83. Lee J, Kim MS, Li R, Liu VY, Fu L, Moore DD, et al. Loss of Bmal1 leads to uncoupling and impaired glucose-stimulated insulin secretion in β-cells. Islets. 2011; 3:381–8.84. Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010; 466:627–31.85. Liu J, Zhou B, Yan M, Huang R, Wang Y, He Z, et al. CLOCK and BMAL1 regulate muscle insulin sensitivity via SIRT1 in male mice. Endocrinology. 2016; 157:2259–69.86. Dyar KA, Ciciliot S, Wright LE, Bienso RS, Tagliazucchi GM, Patel VR, et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol Metab. 2013; 3:29–41.87. Jang H, Lee GY, Selby CP, Lee G, Jeon YG, Lee JH, et al. SREBP1c-CRY1 signalling represses hepatic glucose production by promoting FOXO1 degradation during refeeding. Nat Commun. 2016; 7:12180.88. Lamia KA, Papp SJ, Yu RT, Barish GD, Uhlenhaut NH, Jonker JW, et al. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature. 2011; 480:552–6.89. Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci U S A. 2008; 105:15172–7.90. Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005; 308:1043–5.91. Dashti HS, Follis JL, Smith CE, Tanaka T, Garaulet M, Gottlieb DJ, et al. Gene-environment interactions of circadian-related genes for cardiometabolic traits. Diabetes Care. 2015; 38:1456–66.92. Garcia-Rios A, Gomez-Delgado FJ, Garaulet M, Alcala-Diaz JF, Delgado-Lista FJ, Marin C, et al. Beneficial effect of CLOCK gene polymorphism rs1801260 in combination with low-fat diet on insulin metabolism in the patients with metabolic syndrome. Chronobiol Int. 2014; 31:401–8.93. Corella D, Asensio EM, Coltell O, Sorli JV, Estruch R, Martinez-Gonzalez MA, et al. CLOCK gene variation is associated with incidence of type-2 diabetes and cardiovascular diseases in type-2 diabetic subjects: dietary modulation in the PREDIMED randomized trial. Cardiovasc Diabetol. 2016; 15:4.94. Nankivell VA, Tan JT, Wilsdon LA, Morrison KR, Bilu C, Psaltis PJ, et al. Circadian disruption by short light exposure and a high energy diet impairs glucose tolerance and increases cardiac fibrosis in Psammomys obesus. Sci Rep. 2021; 11:9673.95. Coomans CP, van den Berg SA, Houben T, van Klinken JB, van den Berg R, Pronk AC, et al. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB J. 2013; 27:1721–32.96. Obayashi K, Yamagami Y, Kurumatani N, Saeki K. Bedroom lighting environment and incident diabetes mellitus: a longitudinal study of the HEIJO-KYO cohort. Sleep Med. 2020; 65:1–3.97. Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010; 137:95–101.98. Wu IH, Heredia N, Dong Q, McNeill LH, Balachandran DD, Lu Q, et al. Sleep duration and type 2 diabetes risk: a prospective study in a population-based Mexican American cohort. Sleep Health. 2021; 7:168–76.99. Lee DY, Jung I, Park SY, Yu JH, Seo JA, Kim KJ, et al. Sleep duration and the risk of type 2 diabetes: a community-based cohort study with a 16-year follow-up. Endocrinol Metab (Seoul). 2023; 38:146–55.100. Antza C, Kostopoulos G, Mostafa S, Nirantharakumar K, Tahrani A. The links between sleep duration, obesity and type 2 diabetes mellitus. J Endocrinol. 2021; 252:125–41.101. Han X, Liu B, Wang J, Pan A, Li Y, Hu H, et al. Long sleep duration and afternoon napping are associated with higher risk of incident diabetes in middle-aged and older Chinese: the Dongfeng-Tongji cohort study. Ann Med. 2016; 48:216–23.102. Maskarinec G, Jacobs S, Amshoff Y, Setiawan VW, Shvetsov YB, Franke AA, et al. Sleep duration and incidence of type 2 diabetes: the Multiethnic Cohort. Sleep Health. 2018; 4:27–32.103. Lu H, Yang Q, Tian F, Lyu Y, He H, Xin X, et al. A meta-analysis of a cohort study on the association between sleep duration and type 2 diabetes mellitus. J Diabetes Res. 2021; 2021:8861038.104. Shan Z, Ma H, Xie M, Yan P, Guo Y, Bao W, et al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2015; 38:529–37.105. Cui S, Li Y, Chen Y, Ren P, Fan M, Yang X, et al. Association of sleep duration with risk of type 2 diabetes mellitus in a rural Chinese population: a nested case-control study. Sleep Breath. 2022; 26:2025–33.106. Song Q, Liu X, Zhou W, Wang X, Wu S. Short-term changes in sleep duration and risk of type 2 diabetes: Kailuan prospective study. Medicine (Baltimore). 2016; 95:e5363.107. Stefan L, Sporis G, Kristicevic T, Knjaz D. Associations between sleep quality and its domains and insufficient physical activity in a large sample of Croatian young adults: a crosssectional study. BMJ Open. 2018; 8:e021902.108. Lou P, Zhang P, Zhang L, Chen P, Chang G, Zhang N, et al. Effects of sleep duration and sleep quality on prevalence of type 2 diabetes mellitus: a 5-year follow-up study in China. Diabetes Res Clin Pract. 2015; 109:178–84.109. Kita T, Yoshioka E, Satoh H, Saijo Y, Kawaharada M, Okada E, et al. Short sleep duration and poor sleep quality increase the risk of diabetes in Japanese workers with no family history of diabetes. Diabetes Care. 2012; 35:313–8.110. Seo JA, Lee DY, Yu JH, Cho H, Lee SK, Suh S, et al. Habitual late sleep initiation is associated with increased incidence of type 2 diabetes mellitus in Korean adults: the Korean Genome and Epidemiology Study. Sleep. 2019; 42:zsz090.111. Griggs S, Strohl KP, Grey M, Barbato E, Margevicius S, Hickman RL Jr. Circadian characteristics of the rest-activity rhythm, executive function, and glucose fluctuations in young adults with type 1 diabetes. Chronobiol Int. 2021; 38:1477–87.112. Farabi SS, Carley DW, Quinn L. Glucose variations and activity are strongly coupled in sleep and wake in young adults with type 1 diabetes. Biol Res Nurs. 2017; 19:249–57.113. Reutrakul S, Siwasaranond N, Nimitphong H, Saetung S, Chirakalwasan N, Ongphiphadhanakul B, et al. Relationships among sleep timing, sleep duration and glycemic control in type 2 diabetes in Thailand. Chronobiol Int. 2015; 32:1469–76.114. Xiao Q, Qian J, Evans DS, Redline S, Lane NE, Ancoli-Israel S, et al. Cross-sectional and prospective associations of rest-activity rhythms with metabolic markers and type 2 diabetes in older men. Diabetes Care. 2020; 43:2702–12.115. Xiao Q, Matthews CE, Playdon M, Bauer C. The association between rest-activity rhythms and glycemic markers: the US National Health and Nutrition Examination Survey, 2011-2014. Sleep. 2022; 45:zsab291.116. Dashti HS, Gomez-Abellan P, Qian J, Esteban A, Morales E, Scheer FA, et al. Late eating is associated with cardiometabolic risk traits, obesogenic behaviors, and impaired weight loss. Am J Clin Nutr. 2021; 113:154–61.117. Hatamoto Y, Tanoue Y, Yoshimura E, Matsumoto M, Hayashi T, Ogata H, et al. Delayed eating schedule raises mean glucose levels in young adult males: a randomized controlled crossover trial. J Nutr. 2023; 153:1029–37.118. Kwak J, Jang KA, Kim HR, Kang MS, Lee KW, Shin D. Identifying the associations of nightly fasting duration and meal timing with type 2 diabetes mellitus using data from the 2016-2020 Korea National Health and Nutrition Survey. Nutrients. 2023; 15:1385.119. Ali M, Reutrakul S, Petersen G, Knutson KL. Associations between timing and duration of eating and glucose metabolism: a nationally representative study in the U.S. Nutrients. 2023; 15:729.120. Reutrakul S, Hood MM, Crowley SJ, Morgan MK, Teodori M, Knutson KL, et al. Chronotype is independently associated with glycemic control in type 2 diabetes. Diabetes Care. 2013; 36:2523–9.121. Morse SA, Ciechanowski PS, Katon WJ, Hirsch IB. Isn’t this just bedtime snacking?: the potential adverse effects of nighteating symptoms on treatment adherence and outcomes in patients with diabetes. Diabetes Care. 2006; 29:1800–4.122. Chawla S, Beretoulis S, Deere A, Radenkovic D. The window matters: a systematic review of time restricted eating strategies in relation to cortisol and melatonin secretion. Nutrients. 2021; 13:2525.123. Manoogian EN, Chow LS, Taub PR, Laferrere B, Panda S. Timerestricted eating for the prevention and management of metabolic diseases. Endocr Rev. 2022; 43:405–36.124. Gupta CC, Vincent GE, Coates AM, Khalesi S, Irwin C, Dorrian J, et al. A time to rest, a time to dine: sleep, time-restricted eating, and cardiometabolic health. Nutrients. 2022; 14:420.125. Pellegrini M, Cioffi I, Evangelista A, Ponzo V, Goitre I, Ciccone G, et al. Effects of time-restricted feeding on body weight and metabolism: a systematic review and meta-analysis. Rev Endocr Metab Disord. 2020; 21:17–33.126. Wilkinson MJ, Manoogian EN, Zadourian A, Lo H, Fakhouri S, Shoghi A, et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020; 31:92–104.127. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018; 27:1212–21.128. Parr EB, Steventon-Lorenzen N, Johnston R, Maniar N, Devlin BL, Lim KH, et al. Time-restricted eating improves measures of daily glycaemic control in people with type 2 diabetes. Diabetes Res Clin Pract. 2023; 197:110569.129. Teong XT, Liu K, Vincent AD, Bensalem J, Liu B, Hattersley KJ, et al. Intermittent fasting plus early time-restricted eating versus calorie restriction and standard care in adults at risk of type 2 diabetes: a randomized controlled trial. Nat Med. 2023; 29:963–72.130. Manoogian EN, Zadourian A, Lo HC, Gutierrez NR, Shoghi A, Rosander A, et al. Feasibility of time-restricted eating and impacts on cardiometabolic health in 24-h shift workers: the Healthy Heroes randomized control trial. Cell Metab. 2022; 34:1442–56.131. Wu QJ, Sun H, Wen ZY, Zhang M, Wang HY, He XH, et al. Shift work and health outcomes: an umbrella review of systematic reviews and meta-analyses of epidemiological studies. J Clin Sleep Med. 2022; 18:653–62.132. Maul D. The International Labour Organization: 100 years of global social policy. Berlin: De Gruyter Oldenbourg;2020.133. Vetter C, Dashti HS, Lane JM, Anderson SG, Schernhammer ES, Rutter MK, et al. Night shift work, genetic risk, and type 2 diabetes in the UK Biobank. Diabetes Care. 2018; 41:762–9.134. Li W, Chen Z, Ruan W, Yi G, Wang D, Lu Z. A meta-analysis of cohort studies including dose-response relationship between shift work and the risk of diabetes mellitus. Eur J Epidemiol. 2019; 34:1013–24.135. Gan Y, Yang C, Tong X, Sun H, Cong Y, Yin X, et al. Shift work and diabetes mellitus: a meta-analysis of observational studies. Occup Environ Med. 2015; 72:72–8.136. Morris CJ, Purvis TE, Mistretta J, Scheer FA. Effects of the internal circadian system and circadian misalignment on glucose tolerance in chronic shift workers. J Clin Endocrinol Metab. 2016; 101:1066–74.137. Manodpitipong A, Saetung S, Nimitphong H, Siwasaranond N, Wongphan T, Sornsiriwong C, et al. Night-shift work is associated with poorer glycaemic control in patients with type 2 diabetes. J Sleep Res. 2017; 26:764–72.138. Meule A, Roeser K, Randler C, Kubler A. Skipping breakfast: morningness-eveningness preference is differentially related to state and trait food cravings. Eat Weight Disord. 2012; 17:e304–8.139. Merikanto I, Lahti T, Puolijoki H, Vanhala M, Peltonen M, Laatikainen T, et al. Associations of chronotype and sleep with cardiovascular diseases and type 2 diabetes. Chronobiol Int. 2013; 30:470–7.140. Yu JH, Yun CH, Ahn JH, Suh S, Cho HJ, Lee SK, et al. Evening chronotype is associated with metabolic disorders and body composition in middle-aged adults. J Clin Endocrinol Metab. 2015; 100:1494–502.141. Koopman AD, Rauh SP, van ‘t Riet E, Groeneveld L, van der Heijden AA, Elders PJ, et al. The association between social jetlag, the metabolic syndrome, and type 2 diabetes mellitus in the general population: the New Hoorn Study. J Biol Rhythms. 2017; 32:359–68.142. Bouman EJ, Beulens JW, den Braver NR, Blom MT, Remmelzwaal S, Elders PJ, et al. Social jet lag and (changes in) glycemic and metabolic control in people with type 2 diabetes. Obesity (Silver Spring). 2023; 31:945–54.143. Gonnissen HK, Hursel R, Rutters F, Martens EA, WesterterpPlantenga MS. Effects of sleep fragmentation on appetite and related hormone concentrations over 24 h in healthy men. Br J Nutr. 2013; 109:748–56.144. Fishbein AB, Knutson KL, Zee PC. Circadian disruption and human health. J Clin Invest. 2021; 131:e148286.145. Yang L, Feng H, Chen J, Kwok Wing Y, Benedict C, Tan X, et al. Association of circadian rest-activity rhythms with cardiovascular disease and mortality in type 2 diabetes. Diabetes Res Clin Pract. 2023; 197:110262.146. Ansermet C, Centeno G, Nikolaeva S, Maillard MP, Pradervand S, Firsov D. The intrinsic circadian clock in podocytes controls glomerular filtration rate. Sci Rep. 2019; 9:16089.147. Ansermet C, Centeno G, Bignon Y, Ortiz D, Pradervand S, Garcia A, et al. Dysfunction of the circadian clock in the kidney tubule leads to enhanced kidney gluconeogenesis and exacerbated hyperglycemia in diabetes. Kidney Int. 2022; 101:563–73.148. Solocinski K, Richards J, All S, Cheng KY, Khundmiri SJ, Gumz ML. Transcriptional regulation of NHE3 and SGLT1 by the circadian clock protein Per1 in proximal tubule cells. Am J Physiol Renal Physiol. 2015; 309:F933–42.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Circadian Rhythm Disruption in Cancer Biology: A Review of Literature
- Circadian Rhythm Disruption and Metabolic Syndrome
- Diabetes and Circadian Rhythm
- The Effect of Circadian and Sleep Disruptions on Obesity Risk
- Effect of Disrupted Diurnal Changes on Circadian Clock and Hormonal System: Adverse Health Consequences and Diabetes