Korean Circ J.
2024 Jan;54(1):43-56. 10.4070/kcj.2023.0120.
Entelon150® (Vitis vinifera Seed Extract) Attenuates Degenerative Changes in Intravascular Valve Prostheses in Rabbits
- Affiliations
-
- 1Department of Pediatrics, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- 2Department of Surgery, College of Veterinary Medicine, Jeonbuk National University, Iksan, Korea
- 3Department of Laboratory Animal Medicine, College of Veterinary Medicine, Jeonbuk National University, Iksan, Korea
- 4Department of Physiology, Gachon University College of Medicine, Incheon, Korea
- 5Department of Thoracic and Cardiovascular Surgery, Myoungju Hospital, Yongin, Korea
- KMID: 2550608
- DOI: http://doi.org/10.4070/kcj.2023.0120
Abstract
- Background and Objectives
The therapeutic strategy for inflammation and degenerative calcification is of utmost importance for bioprosthetic heart valve (BHV) implanted patients. The purpose of this study was to compare the anti-inflammatory and anti-calcification effects of Entelon150® (grape seed extract), losartan, and rosuvastatin, in a rabbit model of intravascular BHV leaflet implantation in bovine pericardium.
Methods
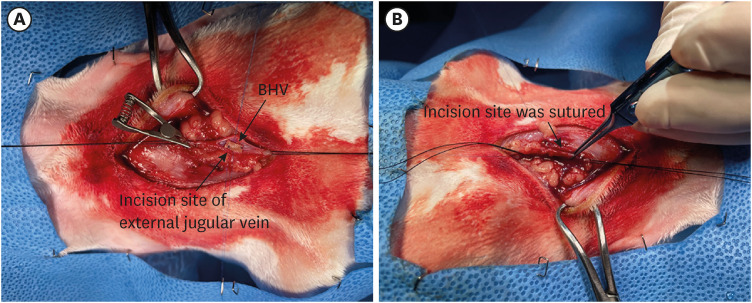
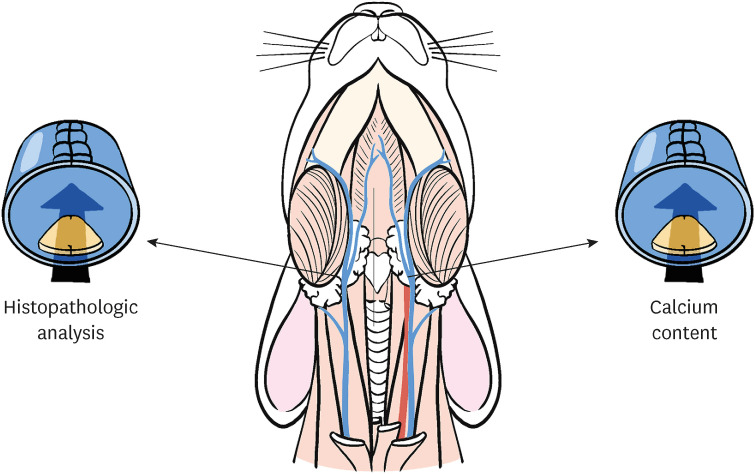
A total of 28 rabbits were implanted with BHV leaflet in the external jugular veins. The Entelon150® group was administered 7.7 mg/kg Entelon150® twice daily for 6 weeks after surgery. The losartan and rosuvastatin groups received 5.14 mg/kg and 1 mg/kg, respectively, once per day. The control group received 1 ml of saline once daily. And then, calcium concentration was measured in the implanted BHV, and histological and molecular analyses were performed on the surrounding tissues.
Results
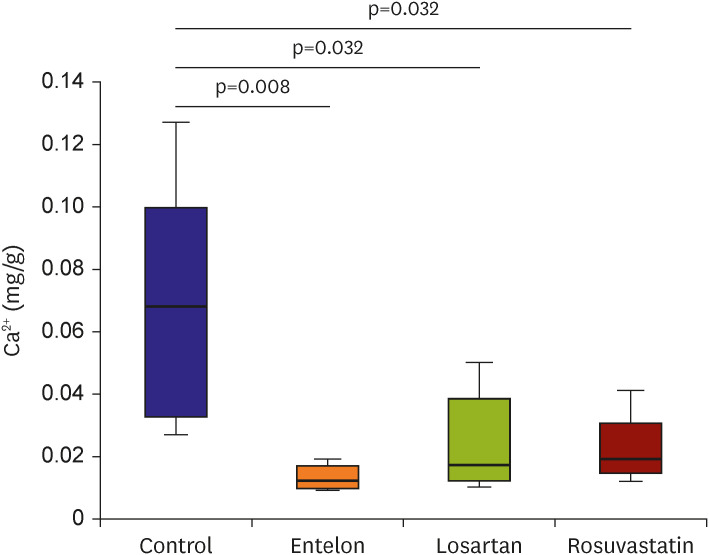
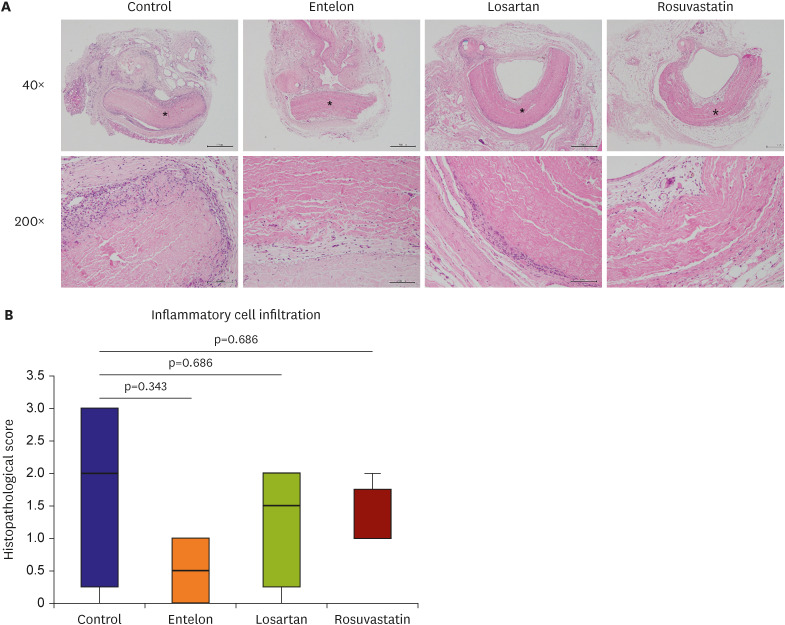
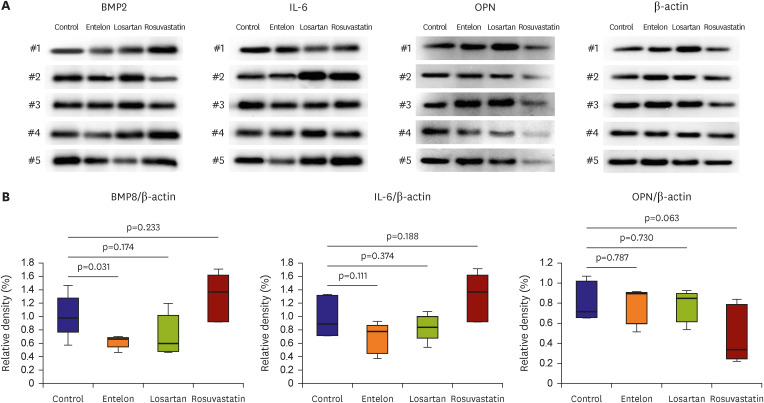
The calcium content of the implanted tissue in the Entelon150® group (0.013±0.004 mg/g) was lower than that in the control group (0.066±0.039 mg/g) (p=0.008). The losartan (0.024±0.016 mg/g, p=0.032) and rosuvastatin (0.022±0.011 mg/g, p=0.032) groups had lower calcium content than the control group, and higher tendency than the Entelon150® group. Immunohistochemistry revealed that the expressions of bone morphogenic protein 2 (BMP2), S-100, and angiotensin II type 1 receptor in the Entelon150® group showed lower tendency than those in the control group. The protein expression levels of BMP2 were reduced in the Entelon150® group compared with those in the control group.
Conclusions
Entelon150® exhibited a significant effect, similar to other drugs, in reducing calcification and inflammation in the intravascular bovine pericardium.
Keyword
Figure
Reference
-
1. Schoen FJ, Levy RJ. Calcification of tissue heart valve substitutes: progress toward understanding and prevention. Ann Thorac Surg. 2005; 79:1072–1080. PMID: 15734452.2. Lee S, Levy RJ, Christian AJ, et al. Calcification and oxidative modifications are associated with progressive bioprosthetic heart valve dysfunction. J Am Heart Assoc. 2017; 6:e005648. PMID: 28483776.3. Kim DH, Park HK, Park YH, et al. Degenerative calcification of pericardial bioprostheses: comparison of five implantation methods in a rabbit model. J Heart Valve Dis. 2015; 24:621–628. PMID: 26897842.4. Colli A, Gherli T, Mestres CA, Pomar JL. Degeneration of native and tissue prosthetic valve in aortic position: do statins play an effective role in prevention? Int J Cardiol. 2007; 116:144–152. PMID: 16828903.5. Côté N, Couture C, Pibarot P, Després JP, Mathieu P. Angiotensin receptor blockers are associated with a lower remodelling score of stenotic aortic valves. Eur J Clin Invest. 2011; 41:1172–1179. PMID: 21988540.6. Aggarwal RK, Showkathali R. Rosuvastatin calcium in acute coronary syndromes. Expert Opin Pharmacother. 2013; 14:1215–1227. PMID: 23574635.7. Lee S, Kim DH, Youn YN, Joo HC, Yoo KJ, Lee SH. Rosuvastatin attenuates bioprosthetic heart valve calcification. J Thorac Cardiovasc Surg. 2019; 158:731–741.e1. PMID: 30738596.8. Choi GC, Kim S, Rahman MM, Oh JH, Cho YS, Shin HJ. Entelon (vitis vinifera seed extract) reduces degenerative changes in bovine pericardium valve leaflet in a dog intravascular implant model. PLoS One. 2021; 16:e0235454. PMID: 33661896.9. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016; 7:27–31. PMID: 27057123.10. Nandakumar V, Singh T, Katiyar SK. Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Lett. 2008; 269:378–387. PMID: 18457915.11. Kaur M, Agarwal C, Agarwal R. Anticancer and cancer chemopreventive potential of grape seed extract and other grape-based products. J Nutr. 2009; 139:1806S–1812S. PMID: 19640973.12. Wang H, Xue Y, Zhang H, et al. Dietary grape seed extract ameliorates symptoms of inflammatory bowel disease in IL10-deficient mice. Mol Nutr Food Res. 2013; 57:2253–2257. PMID: 23963706.13. Zhang Y, Shi H, Wang W, et al. Antithrombotic effect of grape seed proanthocyanidins extract in a rat model of deep vein thrombosis. J Vasc Surg. 2011; 53:743–753. PMID: 21095090.14. Singh RP, Tyagi AK, Dhanalakshmi S, Agarwal R, Agarwal C. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. Int J Cancer. 2004; 108:733–740. PMID: 14696100.15. Dvir D, Bourguignon T, Otto CM, et al. Standardized definition of structural valve degeneration for surgical and transcatheter bioprosthetic aortic valves. Circulation. 2018; 137:388–399. PMID: 29358344.16. Wen S, Zhou Y, Yim WY, et al. Mechanisms and drug therapies of bioprosthetic heart valve calcification. Front Pharmacol. 2022; 13:909801. PMID: 35721165.17. Lorusso R, Corradi D, Maestri R, et al. Atorvastatin attenuates post-implant tissue degeneration of cardiac prosthetic valve bovine pericardial tissue in a subcutaneous animal model. Int J Cardiol. 2010; 141:68–74. PMID: 19167110.18. Eishi K, Ishibashi-Ueda H, Nakano K, et al. Calcific degeneration of bioprosthetic aortic valves in patients receiving steroid therapy. J Heart Valve Dis. 1996; 5:668–672. PMID: 8953446.19. Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. 2007; 21:1387–1394. PMID: 17410187.20. Shin HJ, Kim DH, Park HK, Park YH. The angiotensin II type 1 receptor blocker losartan attenuates bioprosthetic valve leaflet calcification in a rabbit intravascular implant model. Eur J Cardiothorac Surg. 2016; 50:1045–1052. PMID: 27261074.21. Zhang M, Sara JD, Wang FL, et al. Increased plasma BMP-2 levels are associated with atherosclerosis burden and coronary calcification in type 2 diabetic patients. Cardiovasc Diabetol. 2015; 14:64. PMID: 26003174.22. Xia C, Braunstein Z, Toomey AC, Zhong J, Rao X. S100 proteins as an important regulator of macrophage inflammation. Front Immunol. 2018; 8:1908. PMID: 29379499.23. O’Brien KD. Pathogenesis of calcific aortic valve disease: a disease process comes of age (and a good deal more). Arterioscler Thromb Vasc Biol. 2006; 26:1721–1728. PMID: 16709942.24. Latif N, Sarathchandra P, Chester AH, Yacoub MH. Expression of smooth muscle cell markers and co-activators in calcified aortic valves. Eur Heart J. 2015; 36:1335–1345. PMID: 24419809.25. Sica DA, Gehr TW, Ghosh S. Clinical pharmacokinetics of losartan. Clin Pharmacokinet. 2005; 44:797–814. PMID: 16029066.26. Toth PP, Dayspring TD. Drug safety evaluation of rosuvastatin. Expert Opin Drug Saf. 2011; 10:969–986. PMID: 21999163.27. Meuris B, Ozaki S, Herijgers P, Verbeken E, Flameng W. Bioprosthetic tissue calcification: influence of blood contact and arterial pressure. an experimental study in rats and sheep. J Heart Valve Dis. 2003; 12:392–399. PMID: 12803341.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A nationwide study of compliance of venoactive drugs in chronic venous disease patients
- Effects of grape pomace on the antioxidant defense system in diet-induced hypercholesterolemic rabbits
- Neuroprotective effects of Vitis vinifera extract on prediabetic mice induced by a high-fat diet
- Grape seed extract (Vitis vinifera) partially reverses high fat diet-induced obesity in C57BL/6J mice
- Cytoprotective Effect of Polyphenolic Compounds against Oxidative Stress in Cultured Retinal Pigment Epithelial Cells