Korean J Pain.
2024 Jan;37(1):26-33. 10.3344/kjp.23262.
Anti-inflammatory and antinociceptive effects of sitagliptin in animal models and possible mechanisms involved in the antinociceptive activity
- Affiliations
-
- 1Department of Pharmacology and Isfahan Pharmaceutical Sciences Research Center, School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Isfahan, Iran
- 2Department of Pharmacology, Faculty of Medicine, Yasuj University of Medical Sciences, Yasuj, Iran
- KMID: 2550129
- DOI: http://doi.org/10.3344/kjp.23262
Abstract
- Background
Sitagliptin is an antidiabetic drug that inhibits dipeptidyl peptidase-4 enzyme. This study aimed to investigate the antinociceptive and anti-inflammatory effects of sitagliptin in formalin and carrageenan tests and determine the possible mechanism(s) of its antinociceptive activity.
Methods
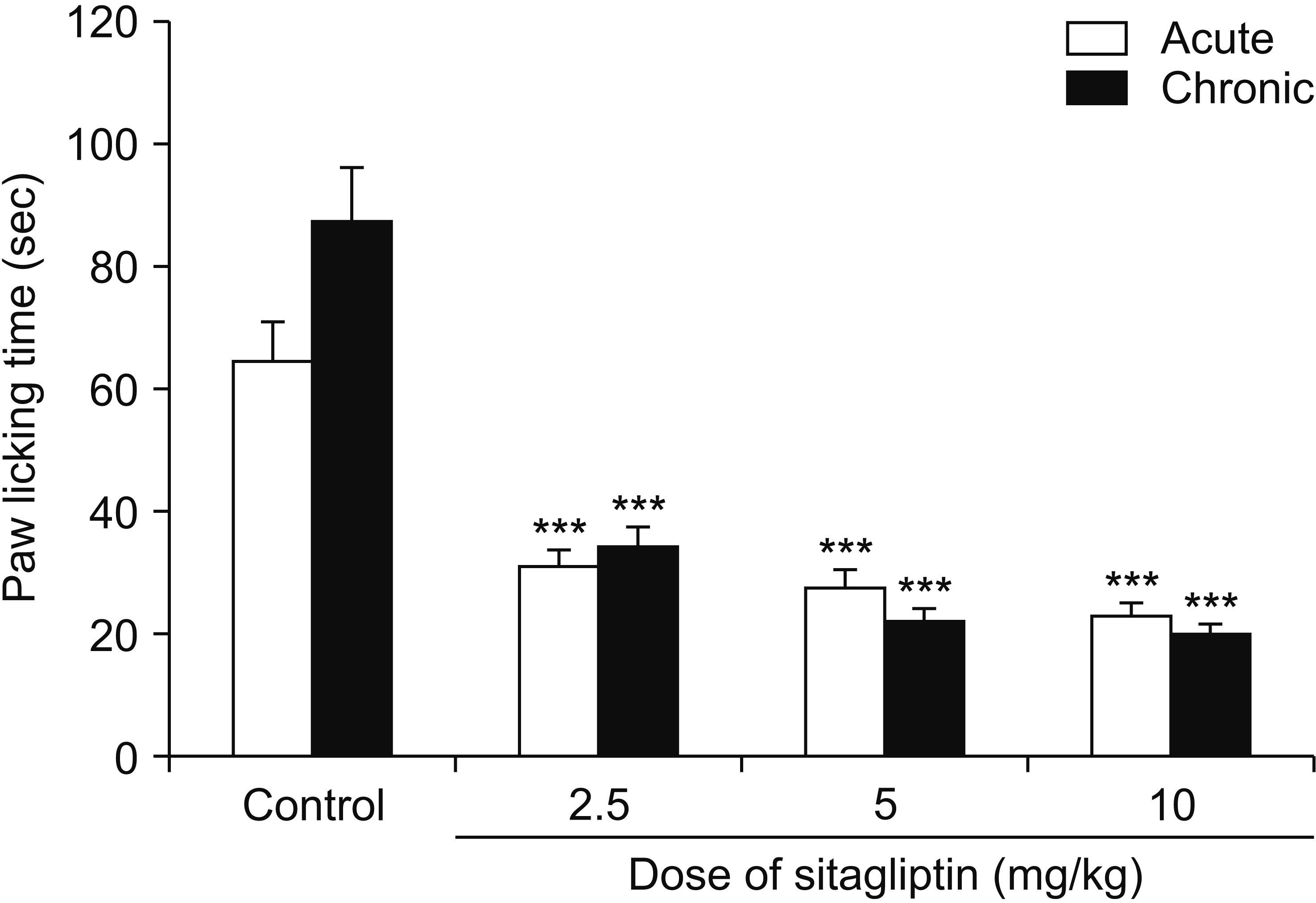
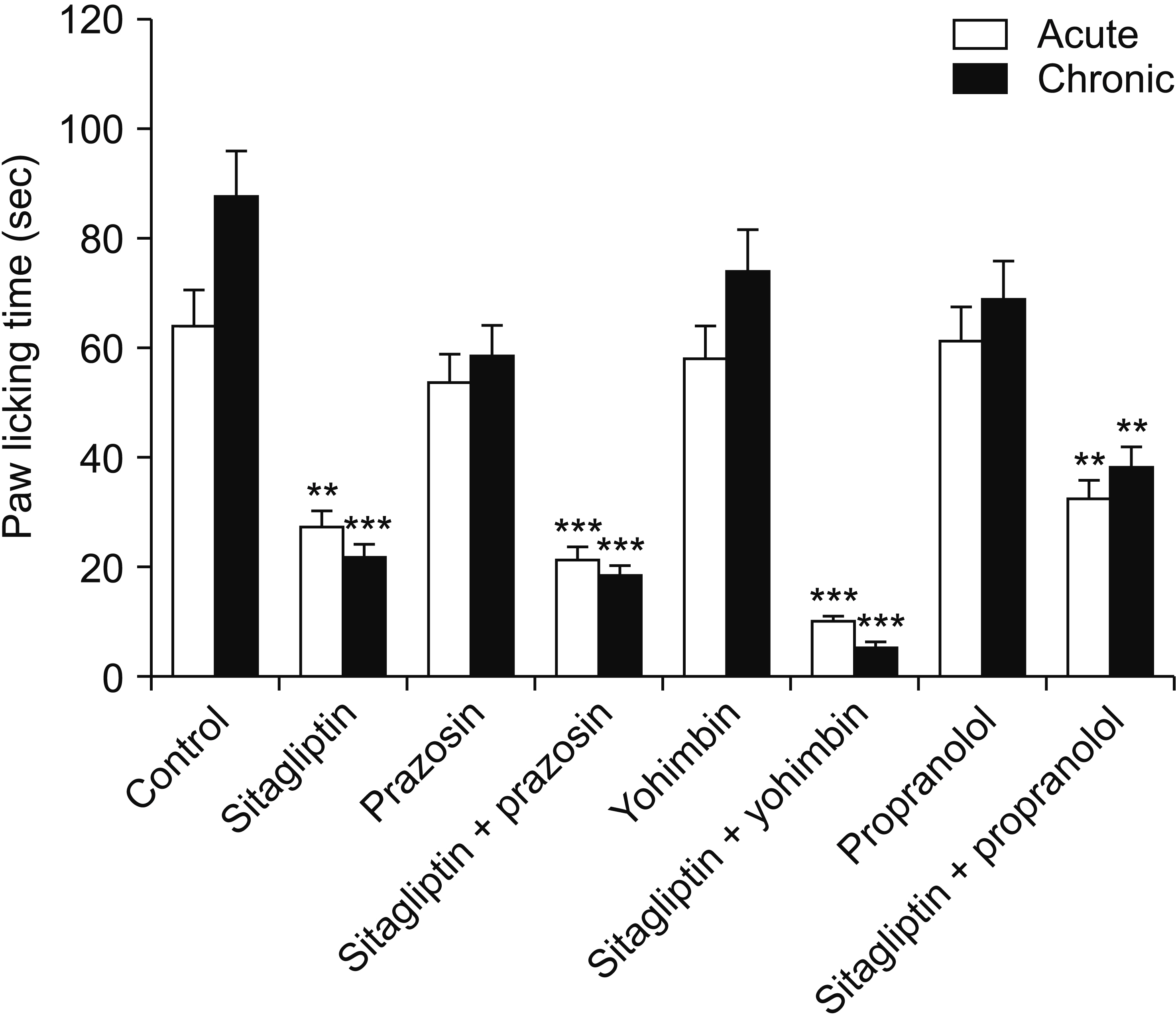
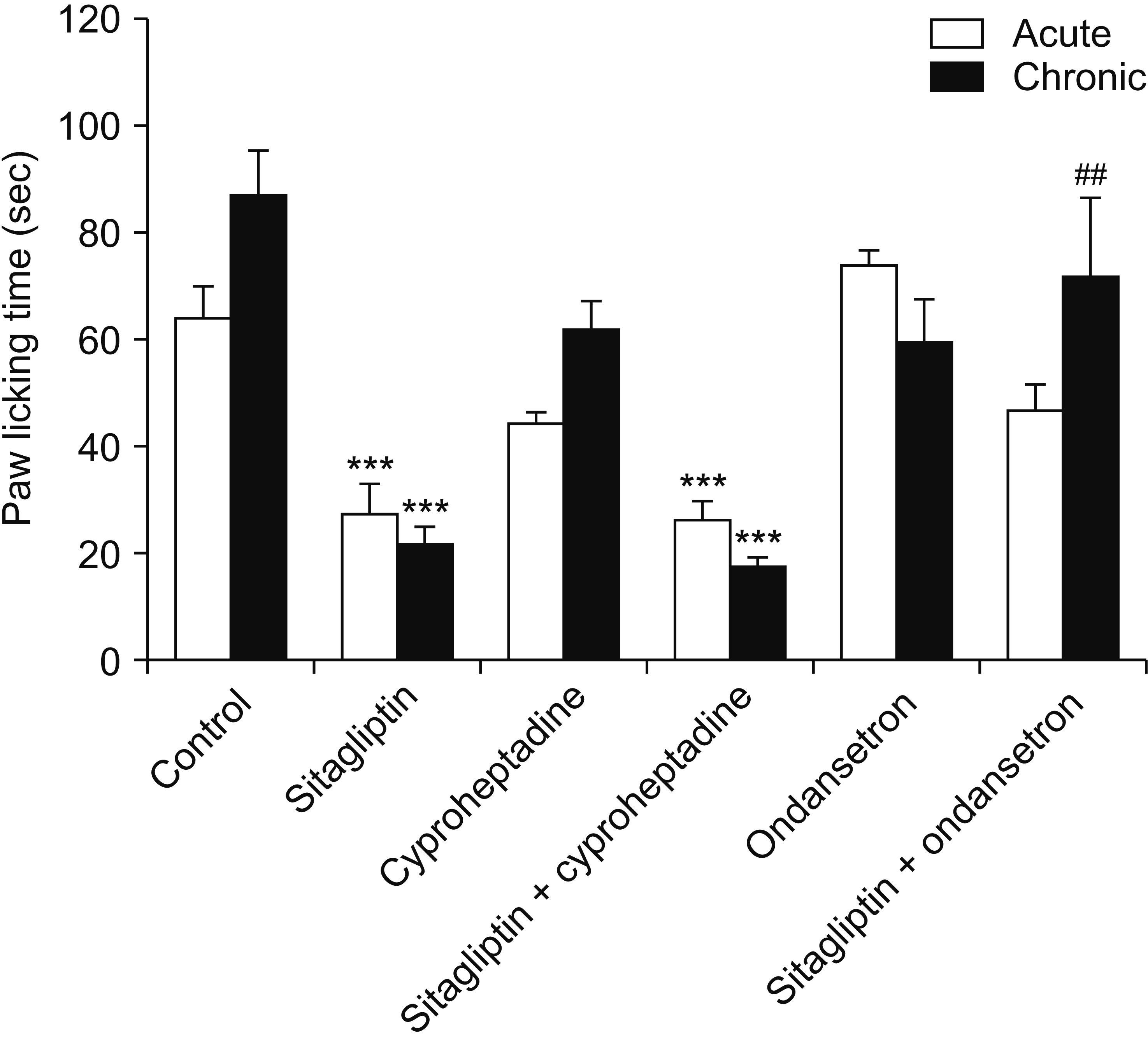
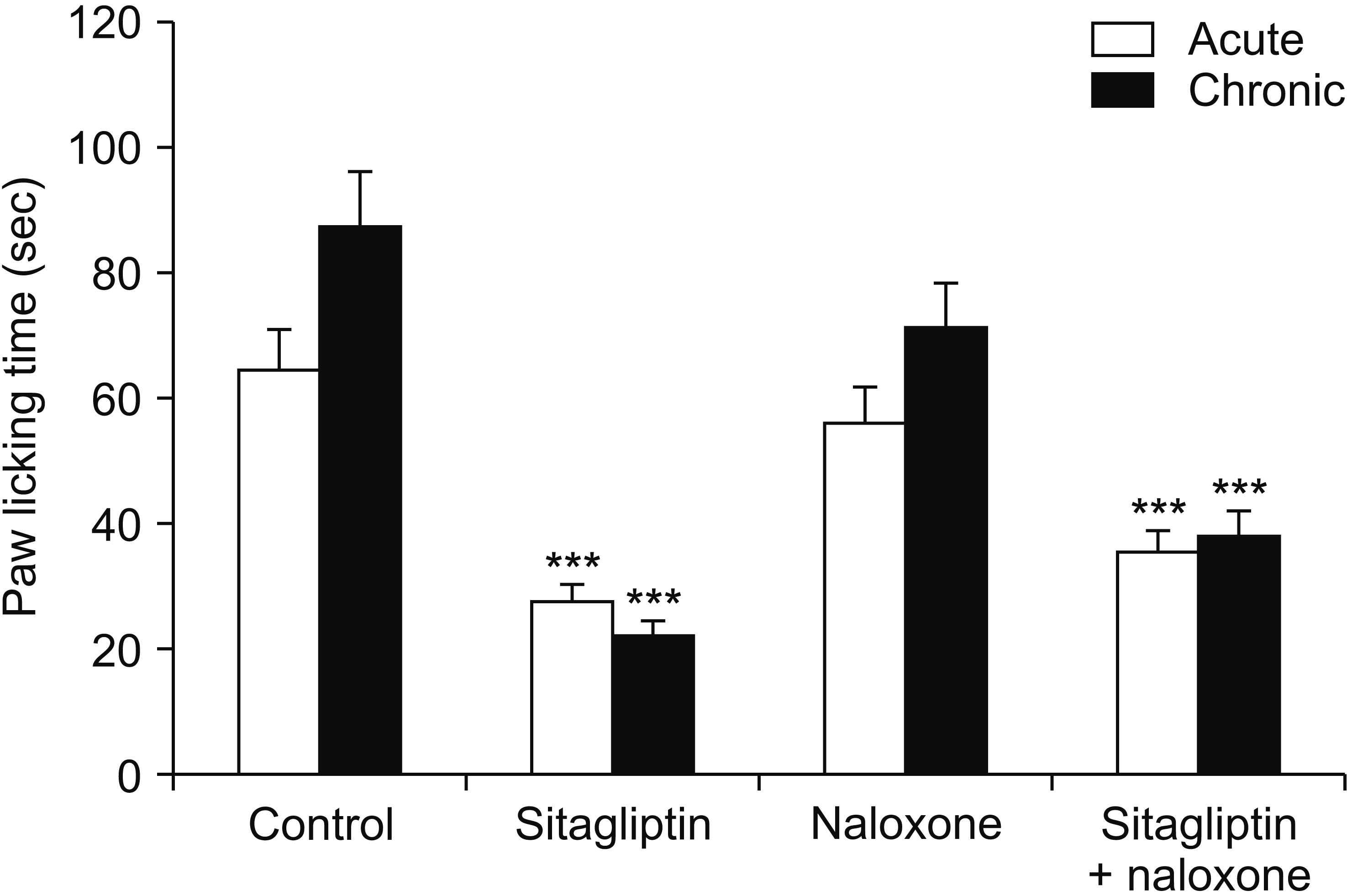
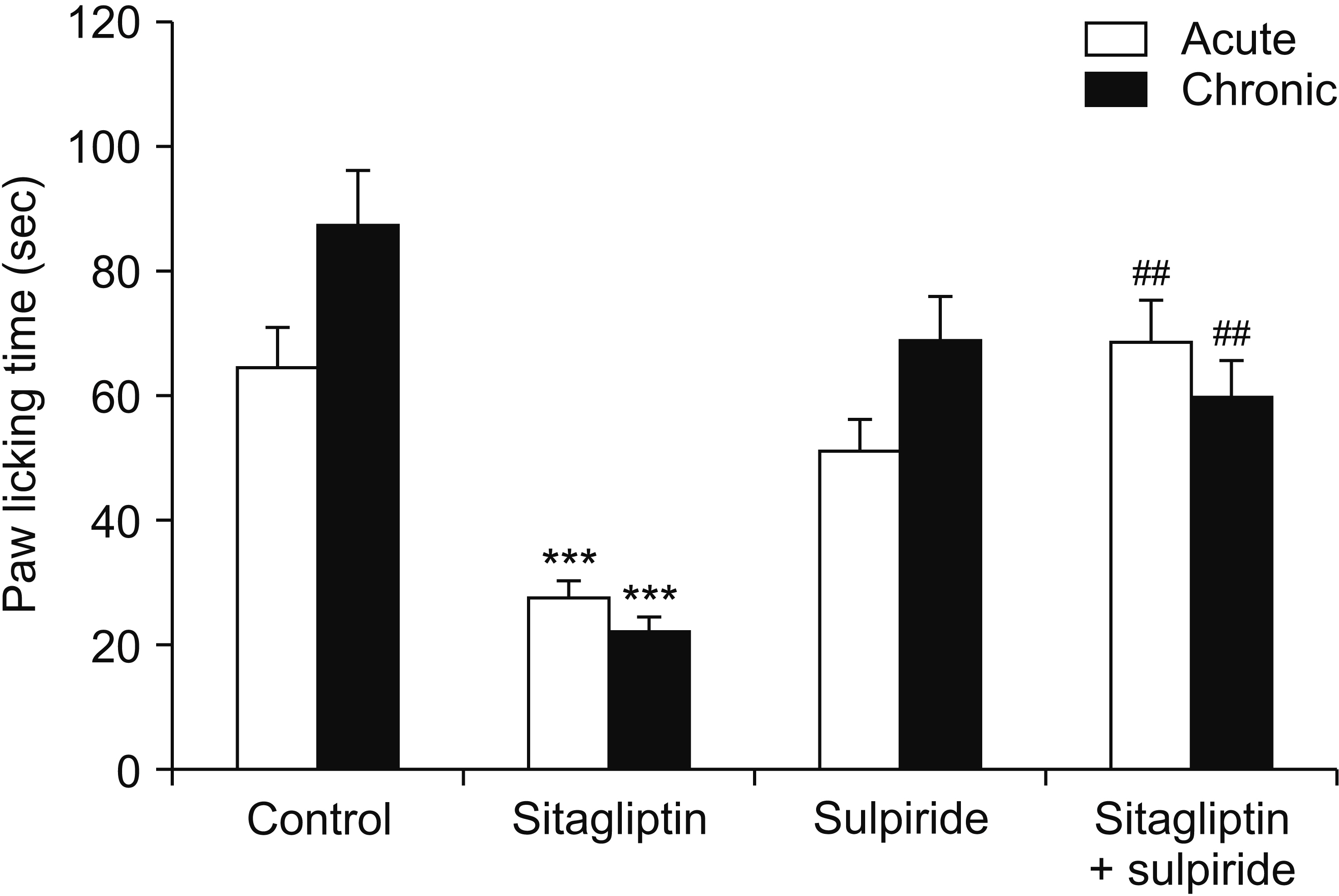
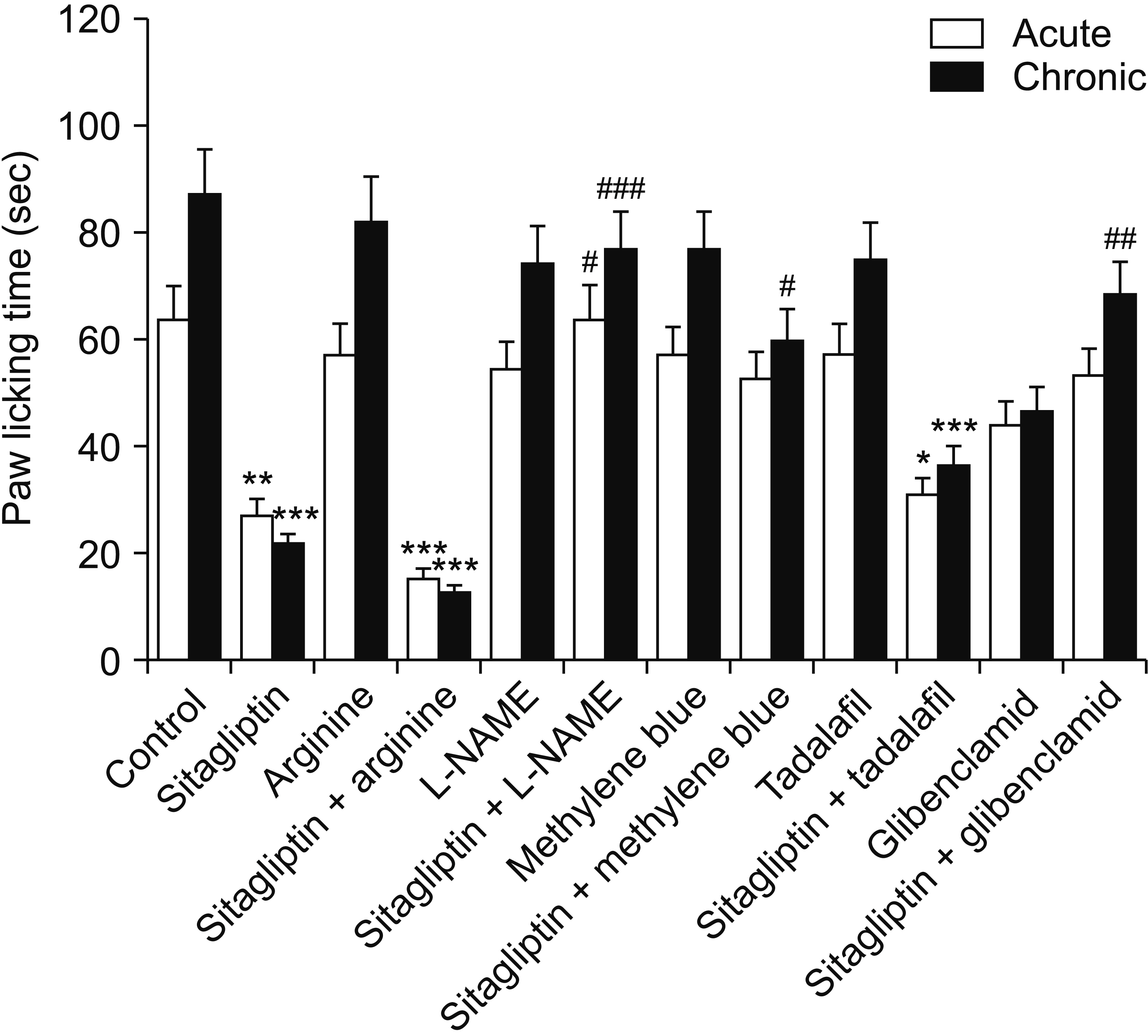
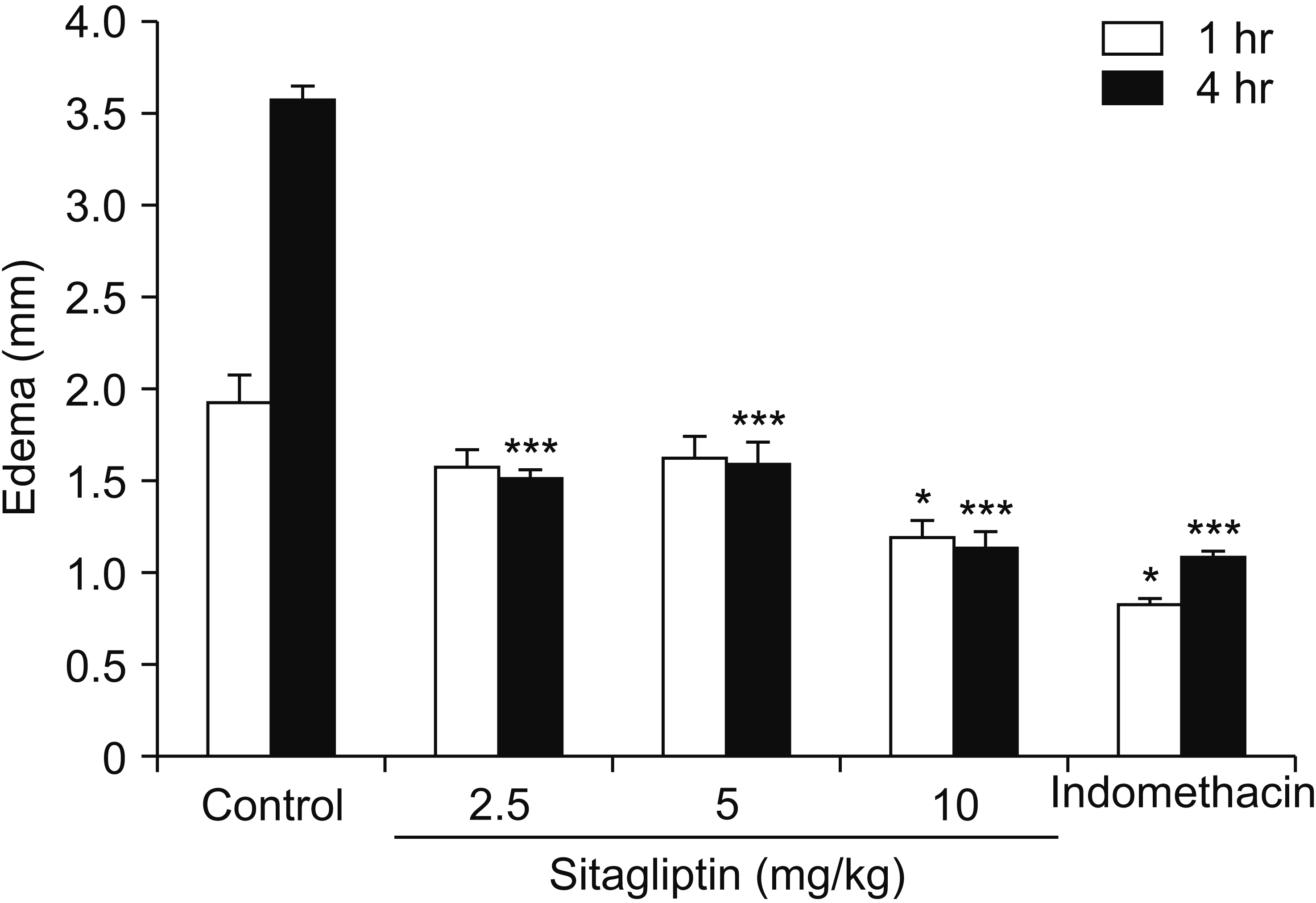
Male Swiss mice (25–30 g) and male Wistar rats (180–220 g) were used for formalin and carrageenan tests, respectively. In the formalin test, paw licking time and in the carrageenan test, paw thickness were considered as indexes of pain behavior and inflammation respectively. Three doses of sitagliptin (2.5, 5, and 10 mg/kg) were used in these tests. Also, several antagonists and enzyme inhibitors were used to evaluate the role of adrenergic, serotonergic, dopaminergic, and opioid receptors as well as the NO/cGMP/K ATP pathway in the antinociceptive effect of sitagliptin (5 mg/kg).
Results
Sitagliptin showed significant antinociceptive and anti-inflammatory effects in the formalin and carrageenan tests respectively. In the carrageenan test, all three doses of sitagliptin significantly (P < 0.001) reduced paw thickness. Pretreatment with yohimbine, prazosin, propranolol, naloxone, and cyproheptadine could not reverse the antinociceptive effect of sitagliptin (5 mg/Kg), which indicates that adrenergic, opioid, and serotonin receptors (5HT 2 ) are not involved in the antinociceptive effects. L-NAME, methylene blue, glibenclamide, ondansetron, and sulpiride were able to reverse this effect.
Conclusions
NO/cGMP/K ATP , 5HT3 and D2 pathways play an important role in the antinociceptive effect of sitagliptin. Additionally significant anti-inflammatory effects observed in the carrageenan test might contribute in reduction of pain response in the second phase of the formalin test.
Keyword
Figure
Cited by 1 articles
-
Analgesic and anti-inflammatory effects of galangin: a potential pathway to inhibit transient receptor potential vanilloid 1 receptor activation
Kaiwen Lin, Datian Fu, Zhongtao Wang, Xueer Zhang, Canyang Zhu
Korean J Pain. 2024;37(2):151-163. doi: 10.3344/kjp.23363.
Reference
-
1. Scott LJ. 2017; Sitagliptin: a review in type 2 diabetes. Drugs. 77:209–24. DOI: 10.1007/s40265-016-0686-9. PMID: 28078647.2. El-Agamy DS, Abo-Haded HM, Elkablawy MA. 2016; Cardioprotective effects of sitagliptin against doxorubicin-induced cardiotoxicity in rats. Exp Biol Med (Maywood). 241:1577–87. DOI: 10.1177/1535370216643418. PMID: 27037281. PMCID: PMC4994902.3. Kelleni MT, Amin EF, Abdelrahman AM. 2015; Effect of metformin and sitagliptin on doxorubicin-induced cardiotoxicity in rats: impact of oxidative stress, inflammation, and apoptosis. J Toxicol. 2015:424813. DOI: 10.1155/2015/424813. PMID: 26880912. PMCID: PMC4736207. PMID: 39871572ab17464d9aa88e7b27da2251.4. Újhelyi J, Újhelyi Z, Szalai A, László JF, Cayasso M, Vecsernyés M, et al. 2014; Analgesic and anti-inflammatory effectiveness of sitagliptin and vildagliptin in mice. Regul Pept. 194-195:23–9. DOI: 10.1016/j.regpep.2014.09.006. PMID: 25229125.5. Kawasaki T, Chen W, Htwe YM, Tatsumi K, Dudek SM. 2018; DPP4 inhibition by sitagliptin attenuates LPS-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 315:L834–45. DOI: 10.1152/ajplung.00031.2018. PMID: 30188745.6. Yam MF, Loh YC, Tan CS, Khadijah Adam S, Abdul Manan N, Basir R. 2018; General pathways of pain sensation and the major neurotransmitters involved in pain regulation. Int J Mol Sci. 19:2164. DOI: 10.3390/ijms19082164. PMID: 30042373. PMCID: PMC6121522. PMID: d4e0c173c2ae4d2b94ca429439af0175.7. Riedel W, Neeck G. 2001; Nociception, pain, and antinociception: current concepts. Z Rheumatol. 60:404–15. DOI: 10.1007/s003930170003. PMID: 11826734.8. Beck TC, Dix TA, Reichel CM. 2019; Targeting peripheral kappa opioid receptors for the treatment of chronic pain: review article. Adv Nanomed Nanotechnol Res. 1:16–9. DOI: 10.4155/fdd-2019-0022. PMID: 31742251. PMCID: PMC6859935. PMID: 73a6f4faefbb4b7cbcbeb4f0116a2faa.9. Bardin L. 2011; The complex role of serotonin and 5-HT receptors in chronic pain. Behav Pharmacol. 22:390–404. DOI: 10.1097/FBP.0b013e328349aae4. PMID: 21808193.10. Pertovaara A. 2013; The noradrenergic pain regulation system: a potential target for pain therapy. Eur J Pharmacol. 716:2–7. DOI: 10.1016/j.ejphar.2013.01.067. PMID: 23500194.11. Wood PB. 2006; Mesolimbic dopaminergic mechanisms and pain control. Pain. 120:230–4. DOI: 10.1016/j.pain.2005.12.014. PMID: 16427195.12. Cury Y, Picolo G, Gutierrez VP, Ferreira SH. 2011; Pain and analgesia: the dual effect of nitric oxide in the nociceptive system. Nitric Oxide. 25:243–54. DOI: 10.1016/j.niox.2011.06.004. PMID: 21723953.13. Florentino IF, Galdino PM, De Oliveira LP, Silva DP, Pazini F, Vanderlinde FA, et al. 2015; Involvement of the NO/cGMP/KATP pathway in the antinociceptive effect of the new pyrazole 5-(1-(3-fluorophenyl)-1H-pyrazol-4-yl)-2H-tetrazole (LQFM-021). Nitric Oxide. 47:17–24. DOI: 10.1016/j.niox.2015.02.146. PMID: 25754796.14. Hajhashemi V, Salimian M, Hajihashemi O. 2023; Involvement of the NO/cGMP/K ATP pathway in the antinociceptive effect of rosemary (Rosmarinus officinalis) essential oil in mice. Behav Pharmacol. 34:37–44. DOI: 10.1097/FBP.0000000000000709. PMID: 36730811.15. Pecikoza U, Micov A, Tomić M, Stepanović-Petrović R. 2018; Eslicarbazepine acetate reduces trigeminal nociception: possible role of adrenergic, cholinergic and opioid receptors. Life Sci. 214:167–75. DOI: 10.1016/j.lfs.2018.10.059. PMID: 30393024.16. Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. 1992; The formalin test: an evaluation of the method. Pain. 51:5–17. DOI: 10.1016/0304-3959(92)90003-T. PMID: 1454405.17. Hajhashemi V, Khodarahmi G, Asadi P, Rajabi H. 2022; Evaluation of the antinociceptive effects of a selection of triazine derivatives in mice. Korean J Pain. 35:440–6. DOI: 10.3344/kjp.2022.35.4.440. PMID: 36175343. PMCID: PMC9530681.18. Morris CJ. 2003; Carrageenan-induced paw edema in the rat and mouse. Methods Mol Biol. 225:115–21. DOI: 10.1385/1-59259-374-7:115. PMID: 12769480.19. Vazquez E, Navarro M, Salazar Y, Crespo G, Bruges G, Osorio C, et al. 2015; Systemic changes following carrageenan-induced paw inflammation in rats. Inflamm Res. 64:333–42. DOI: 10.1007/s00011-015-0814-0. PMID: 25772383.20. Savegnago L, Jesse CR, Santos AR, Rocha JB, Nogueira CW. 2008; Mechanisms involved in the antinociceptive effect caused by diphenyl diselenide in the formalin test. J Pharm Pharmacol. 60:1679–86. DOI: 10.1211/jpp.60.12.0015. PMID: 19000374.21. Viguier F, Michot B, Hamon M, Bourgoin S. 2013; Multiple roles of serotonin in pain control mechanisms--implications of 5-HT₇ and other 5-HT receptor types. Eur J Pharmacol. 716:8–16. DOI: 10.1016/j.ejphar.2013.01.074. PMID: 23500207.22. Kapur S, Zipursky RB, Jones C, Wilson AA, DaSilva JD, Houle S. 1997; Cyproheptadine: a potent in vivo serotonin antagonist. Am J Psychiatry. 154:884. DOI: 10.1176/ajp.154.6.884a. PMID: 9167527.23. Tan JQ, Fan CK, Cui J, Xu B. 1990; [Analgesic and antipyretic effects of cyproheptadine]. Zhongguo Yao Li Xue Bao. 11:204–7. Chinese. . DOI: 10.32388/2r62yt. PMID: 2087993.24. Vale C, Oliveira F, Assunção J, Fontes-Ribeiro C, Pereira F. 2011; Co-administration of ondansetron decreases the analgesic efficacy of tramadol in humans. Pharmacology. 88:182–7. DOI: 10.1159/000330740. PMID: 21952250.25. Benarroch EE. 2016; Involvement of the nucleus accumbens and dopamine system in chronic pain. Neurology. 87:1720–6. DOI: 10.1212/WNL.0000000000003243. PMID: 27655737.26. Wood PB. 2008; Role of central dopamine in pain and analgesia. Expert Rev Neurother. 8:781–97. DOI: 10.1586/14737175.8.5.781. PMID: 18457535.27. Morgan MJ, Franklin KB. 1991; Dopamine receptor subtypes and formalin test analgesia. Pharmacol Biochem Behav. 40:317–22. DOI: 10.1016/0091-3057(91)90560-O. PMID: 1687167.28. Miclescu A, Gordh T. 2009; Nitric oxide and pain: 'something old, something new'. Acta Anaesthesiol Scand. 53:1107–20. DOI: 10.1111/j.1399-6576.2009.02054.x. PMID: 19702699.29. Kawabata A, Manabe S, Manabe Y, Takagi H. 1994; Effect of topical administration of L-arginine on formalin-induced nociception in the mouse: a dual role of peripherally formed NO in pain modulation. Br J Pharmacol. 112:547–50. DOI: 10.1111/j.1476-5381.1994.tb13108.x. PMID: 7521259. PMCID: PMC1910365.30. Bulutcu F, Dogrul A, Güç MO. 2002; The involvement of nitric oxide in the analgesic effects of ketamine. Life Sci. 71:841–53. DOI: 10.1016/S0024-3205(02)01765-4. PMID: 12074943.31. Ortiz MI, Granados-Soto V, Castañeda-Hernández G. 2003; The NO-cGMP-K+ channel pathway participates in the antinociceptive effect of diclofenac, but not of indomethacin. Pharmacol Biochem Behav. 76:187–95. DOI: 10.1016/S0091-3057(03)00214-4. PMID: 13679232.32. Lázaro-Ibáñez GG, Torres-López JE, Granados-Soto V. 2001; Participation of the nitric oxide-cyclic GMP-ATP-sensitive K(+) channel pathway in the antinociceptive action of ketorolac. Eur J Pharmacol. 426:39–44. DOI: 10.1016/S0014-2999(01)01206-7. PMID: 11525769.33. Sakurada C, Sugiyama A, Nakayama M, Yonezawa A, Sakurada S, Tan-No K, et al. 2001; Antinociceptive effect of spinally injected L-NAME on the acute nociceptive response induced by low concentrations of formalin. Neurochem Int. 38:417–23. DOI: 10.1016/S0197-0186(00)00110-8. PMID: 11222922.34. Babbedge RC, Hart SL, Moore PK. 1993; Anti-nociceptive activity of nitric oxide synthase inhibitors in the mouse: dissociation between the effect of L-NAME and L-NMMA. J Pharm Pharmacol. 45:77–9. DOI: 10.1111/j.2042-7158.1993.tb03686.x. PMID: 7679442.35. Alves DP, Soares AC, Francischi JN, Castro MS, Perez AC, Duarte ID. 2004; Additive antinociceptive effect of the combination of diazoxide, an activator of ATP-sensitive K+ channels, and sodium nitroprusside and dibutyryl-cGMP. Eur J Pharmacol. 489:59–65. DOI: 10.1016/j.ejphar.2004.02.022. PMID: 15063156.36. Yamazumi I, Okuda T, Koga Y. 2001; Involvement of potassium channels in spinal antinociceptions induced by fentanyl, clonidine and bethanechol in rats. Jpn J Pharmacol. 87:268–76. DOI: 10.1254/jjp.87.268. PMID: 11829146.37. De Paz-Campos MA, Chávez-Piña AE, Ortiz MI, Castañeda-Hernández G. 2012; Evidence for the participation of ATP-sensitive potassium channels in the antinociceptive effect of curcumin. Korean J Pain. 25:221–7. DOI: 10.3344/kjp.2012.25.4.221. PMID: 23091682. PMCID: PMC3468798.38. Makdissi A, Ghanim H, Vora M, Green K, Abuaysheh S, Chaudhuri A, et al. 2012; Sitagliptin exerts an antinflammatory action. J Clin Endocrinol Metab. 97:3333–41. DOI: 10.1210/jc.2012-1544. PMID: 22745245. PMCID: PMC3431580.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antinociceptive Effects of Amikacin on Neuropathic Pain in Rats
- Antinociceptive Effects of Tramadol on the Neuropathic Pain in Rats
- Evaluation of the antinociceptive effects of a selection of triazine derivatives in mice
- Antinociceptive and anti-inflammatory effects of N-acetylcysteine and verapamil in Wistar rats
- Antinociception Effect and Mechanisms of Campanula Punctata Extract in the Mouse