Restor Dent Endod.
2021 Aug;46(3):e33. 10.5395/rde.2021.46.e33.
Enhanced visualization of the root canal morphology using a chitosanbased endo-radiopaque solution
- Affiliations
-
- 1Department of Conservative Dentistry and Endodontics, Institute of Dental Sciences, Siksha ‘O’ Anusandhan Deemed to be University, Odisha, India
- 2Department of Conservative Dentistry and Endodontics, Sriram Chandra Bhanja Dental College & Hospital, Utkal University, Odisha, India
- 3Department of Conservative Dentistry and Endodontics, Hi-Tech Dental College & Hospital, Utkal University, Odisha, India
- 4Adult Restorative Dentistry - Endodontics, Oman Dental College, University Complutense of Madrid, Oman, Sultanate of Oman
- KMID: 2548077
- DOI: http://doi.org/10.5395/rde.2021.46.e33
Abstract
Objectives
This study aimed to investigate the efficacy of ionic and non-ionic-based contrast media (in vitro study) and the combinatorial effect of chitosan-based endo-radiopaque solution (CERS) (in vivo study) for visualization of the root canal anatomy.
Materials and Methods
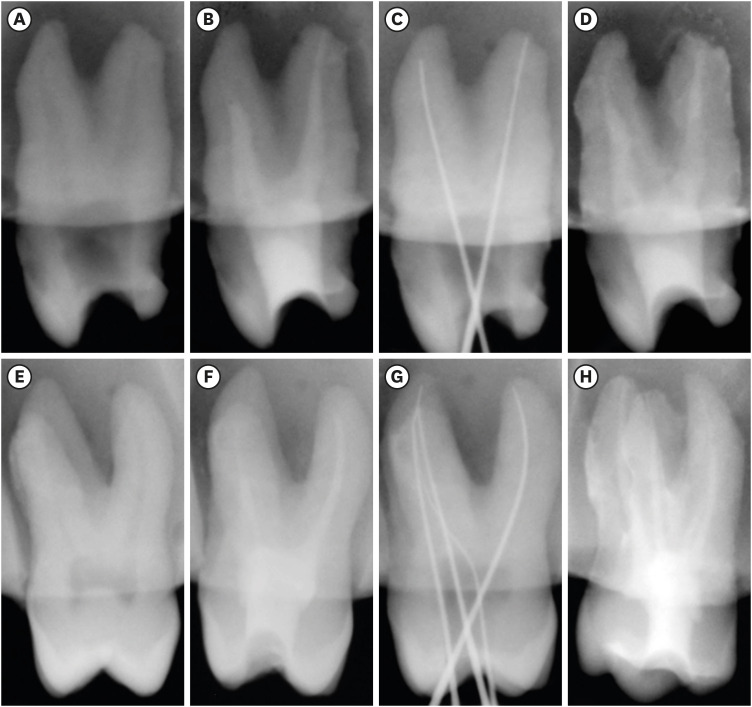
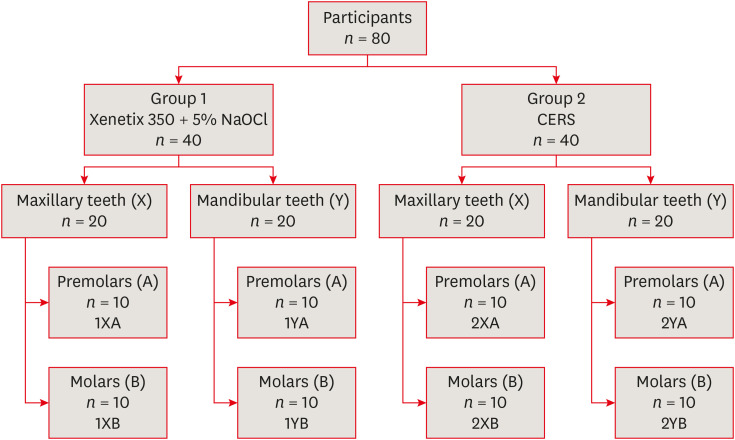
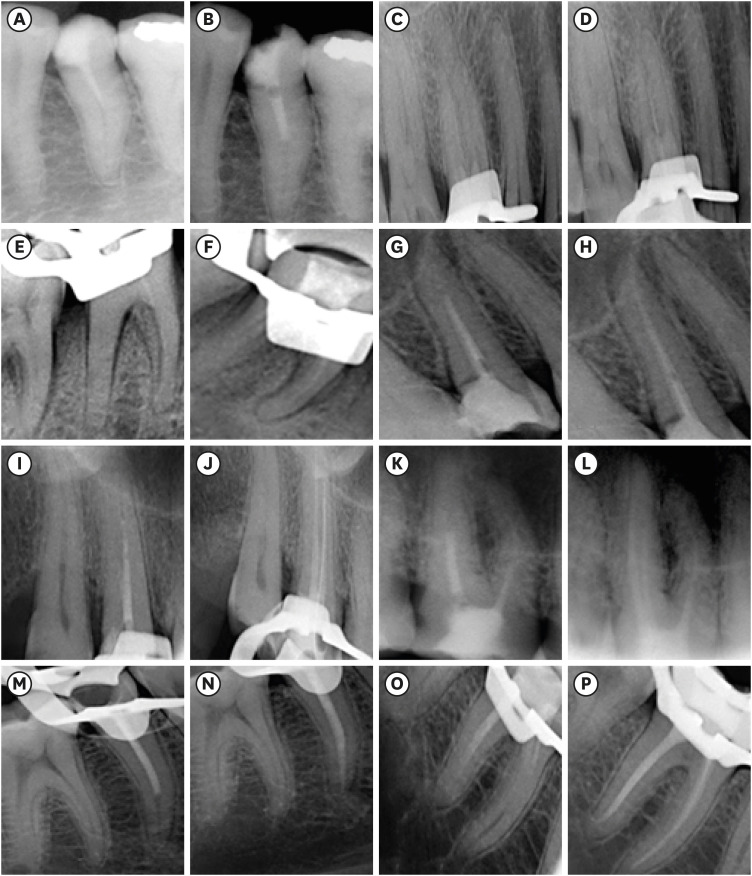
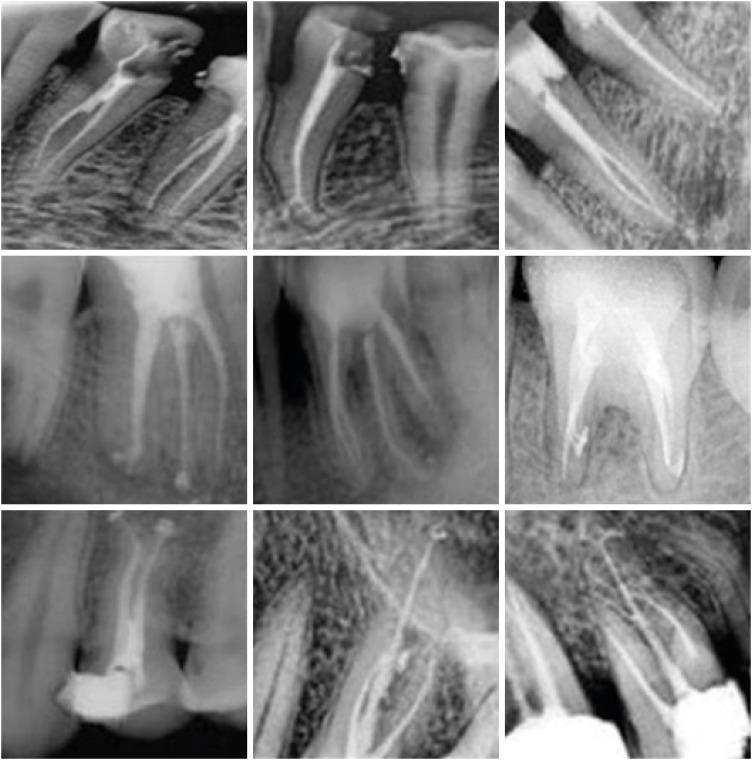
in vitro study (120 teeth): The root canal of maxillary premolars and molars (in vitro group 1 and 2 respectively, n = 60 each) were analyzed using 4 different contrast media (subgroups: Omnipaque 350, Iopamidol, Xenetix 350, and Urografin 76; n = 15 each) in combination with 5.25% sodium hypochlorite (NaOCl). Based on the results of the in vitro study, in vivo study (80 teeth) was done to compare Xenetix 350 + 5.25% NaOCl with CERS (in vivo group 1 and 2 respectively, n = 40 each) on maxillary and mandibular premolars and molars. Two endodontists used radiovisiography to assess the depth of ingress and identify the aberrant root anatomy after access cavity preparation, and after initial cleaning and shaping of canals. Kruskal-Wallis test was used for in vitro comparison (p < 0.05), and Wilcoxon signed-rank test and Mann-Whitney U test for in vivo analysis (p < 0.01).
Results
In vitro study, Xenetix 350 + 5.25% NaOCl facilitated a significant higher visualization (p < 0.05). For in vivo study, CERS had a statistically significant depth of ingress (p < 0.01), and was efficient in identifying the aberrant root canal anatomy of premolars and molars.
Conclusions
CERS facilitates better visualization of the root canal anatomy of human premolars and molars.
Figure
Reference
-
1. Vertucci FJ. Root canal morphology and its relationship to endodontic procedures. Endod Topics. 2005; 10:3–29.
Article2. Cleghorn BM, Christie WH, Dong CC. The root and root canal morphology of the human mandibular first premolar: a literature review. J Endod. 2007; 33:509–516. PMID: 17437863.
Article3. Sachdeva GS, Ballal S, Gopikrishna V, Kandaswamy D. Endodontic management of a mandibular second premolar with four roots and four root canals with the aid of spiral computed tomography: a case report. J Endod. 2008; 34:104–107. PMID: 18155506.
Article4. Barker BC, Parsons KC, Mills PR, Williams GL. Anatomy of root canals. I. Permanent incisors, canines and premolars. Aust Dent J. 1973; 18:320–327. PMID: 4524851.5. Hession RW. Endodontic morphology. I. An alternative method of study. Oral Surg Oral Med Oral Pathol. 1977; 44:456–462. PMID: 269341.6. Lowman JV, Burke RS, Pelleu GB. Patent accessory canals: incidence in molar furcation region. Oral Surg Oral Med Oral Pathol. 1973; 36:580–584. PMID: 4517107.
Article7. Mahale US, Hegde MN, Shetty A, Patil A. Iopamidol in comparison with meglumine sodium diatrizoate in different concentrations as contrast media in clinical endodontics – in vivo study. Indian J Appl Res. 2013; 3:410–413.
Article8. Ahmed HM. Guidelines to enhance the interpretation of two-dimensional periapical radiographic images in endodontics. Eur J Gen Dent. 2015; 4:106.
Article9. Del Carpio-Perochena A, Bramante CM, Duarte MA, de Moura MR, Aouada FA, Kishen A. Chelating and antibacterial properties of chitosan nanoparticles on dentin. Restor Dent Endod. 2015; 40:195–201. PMID: 26295022.
Article10. Goy RC, Britto DD, Assis OB. A review of the antimicrobial activity of chitosan. Polímeros. 2009; 19:241–247.
Article11. Rabea EI, Badawy ME, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 2003; 4:1457–1465. PMID: 14606868.
Article12. Fatimi A, Chabrot P, Berrahmoune S, Coutu JM, Soulez G, Lerouge S. A new injectable radiopaque chitosan-based sclerosing embolizing hydrogel for endovascular therapies. Acta Biomater. 2012; 8:2712–2721. PMID: 22487932.
Article13. Zehtabi F, Dumont-Mackay V, Fatimi A, Bertrand-Grenier A, Héon H, Soulez G, Lerouge S. Chitosan–sodium tetradecyl sulfate hydrogel: characterization and preclinical evaluation of a novel sclerosing embolizing agent for the treatment of endoleaks. Cardiovasc Intervent Radiol. 2017; 40:576–584. PMID: 28078379.
Article14. G ShashirekhaA Jena. Institute of Dental Sciences, Siksha ‘O’ Anusandhan Deemed to be University. Endo-radiopaque composition for visualizing the complex root canal anatomy. India patent. IN 334776. 2020. 3. 16.15. Aditya S, Mithra H, Divya T, Aum J, Darshana D. Study on the efficacy of Iodine based contrast media for interpretation of root canal anatomy. Int Res J Pharm. 2013; 4:207–210.
Article16. Naoum HJ, Love RM, Chandler NP, Herbison P. Effect of X-ray beam angulation and intraradicular contrast medium on radiographic interpretation of lower first molar root canal anatomy. Int Endod J. 2003; 36:12–19. PMID: 12656509.
Article17. Fournier PJ, Steinbrich W, Freitag P, Voegeli E. Evaluation of the clinical safety and efficacy of iobitridol (Xenetix) in intravenous urography. Eur J Radiol. 1996; 23:185–189. PMID: 9003922.
Article18. Lefevre T, Funck F, Aliot E, Ethevenot B. Safety and efficacy of the new iodinated nonionic low-osmolality contrast medium Iobitridol (Xenetix) in coronary and ventricular angiography. Acta Radiol Suppl. 1996; 400:75–80. PMID: 8619358.19. Rossignol AM, Lusson JR, Chantepie A, Losay J. Safety and efficacy of Xenetix, a new iodinated contrast agent, in pediatric angiocardiography. Acta Radiol Suppl. 1996; 400:81–84. PMID: 8619360.20. Jena A, Sahoo SK, Govind S. Root canal irrigants: a review of their interactions, benefits, and limitations. Compend Contin Educ Dent. 2015; 36:256–261. PMID: 25821937.21. Pattanshetti N, Gaidhane M, Al Kandari AM. Root and canal morphology of the mesiobuccal and distal roots of permanent first molars in a Kuwait population--a clinical study. Int Endod J. 2008; 41:755–762. PMID: 18637850.
Article22. Peiris HR, Pitakotuwage TN, Takahashi M, Sasaki K, Kanazawa E. Root canal morphology of mandibular permanent molars at different ages. Int Endod J. 2008; 41:828–835. PMID: 18822010.
Article23. Reis AG, Grazziotin-Soares R, Barletta FB, Fontanella VR, Mahl CR. Second canal in mesiobuccal root of maxillary molars is correlated with root third and patient age: a cone-beam computed tomographic study. J Endod. 2013; 39:588–592. PMID: 23611373.
Article24. Tang MP, Stock CJ. An in vitro method for comparing the effects of different root canal preparation techniques on the shape of curved root canals. Int Endod J. 1989; 22:49–54. PMID: 2599661.
Article25. Silva PV, Guedes DF, Nakadi FV, Pécora JD, Cruz-Filho AM. Chitosan: a new solution for removal of smear layer after root canal instrumentation. Int Endod J. 2013; 46:332–338. PMID: 22970844.
Article26. Bronnec F, Bouillaguet S, Machtou P. Ex vivo assessment of irrigant penetration and renewal during the cleaning and shaping of root canals: a digital subtraction radiographic study. Int Endod J. 2010; 43:275–282. PMID: 20487446.
Article27. Bronnec F, Bouillaguet S, Machtou P. Ex vivo assessment of irrigant penetration and renewal during the final irrigation regimen. Int Endod J. 2010; 43:663–672. PMID: 20491986.
Article28. Munoz HR, Camacho-Cuadra K. In vivo efficacy of three different endodontic irrigation systems for irrigant delivery to working length of mesial canals of mandibular molars. J Endod. 2012; 38:445–448. PMID: 22414827.
Article29. Alonso-Ezpeleta LO, Gasco-Garcia C, Castellanos-Cosano L, Martín-González J, López-Frías FJ, Segura-Egea JJ. Postoperative pain after one-visit root-canal treatment on teeth with vital pulps: comparison of three different obturation techniques. Med Oral Patol Oral Cir Bucal. 2012; 17:e721–e727. PMID: 22322522.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Observation of mandibular second molar roots and root canal morphology using dental cone-beam computed tomography
- A Study of Root Canals Morphology in Primary Molars using Computerized Tomography
- Age-dependent root canal instrumentation techniques: a comprehensive narrative review
- Impact of root canal curvature and instrument type on the amount of extruded debris during retreatment
- Diagnosis and treatment of maxillary molar with abnormality