Cancer Res Treat.
2023 Oct;55(4):1321-1336. 10.4143/crt.2022.1532.
Favorable Immunotherapy Plus Tyrosine Kinase Inhibition Outcome of Renal Cell Carcinoma Patients with Low CDK5 Expression
- Affiliations
-
- 1Department of Urology, Zhongshan Hospital, Fudan University, Shanghai, China
- 2Department of Critical Care Medicine, Zhongshan Hospital, Fudan University, Shanghai, China
- 3School of Life Sciences, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China
- KMID: 2547806
- DOI: http://doi.org/10.4143/crt.2022.1532
Abstract
- Purpose
Immunotherapy (IO) plus tyrosine kinase inhibitor (TKI) has become the first-line treatment for advanced renal cell carcinoma, despite the lack of prognostic biomarkers. Cyclin-dependent kinase 5 (CDK5) affects the tumor microenvironment, which may influence the efficacy of TKI+IO.
Materials and Methods
Two cohorts from our center (Zhongshan Metastatic Renal Cell Carcinoma [ZS-MRCC] cohort, Zhongshan High-risk Localized Renal Cell Carcinoma [ZS-HRRCC] cohort) and one cohort from a clinical trial (JAVELIN-101) were enrolled. The expression of CDK5 of each sample was determined by RNA sequencing. Immune infiltration and T cell function were evaluated by flow cytometry and immunohistochemistry. Response and progression-free survival (PFS) were set as primary endpoints.
Results
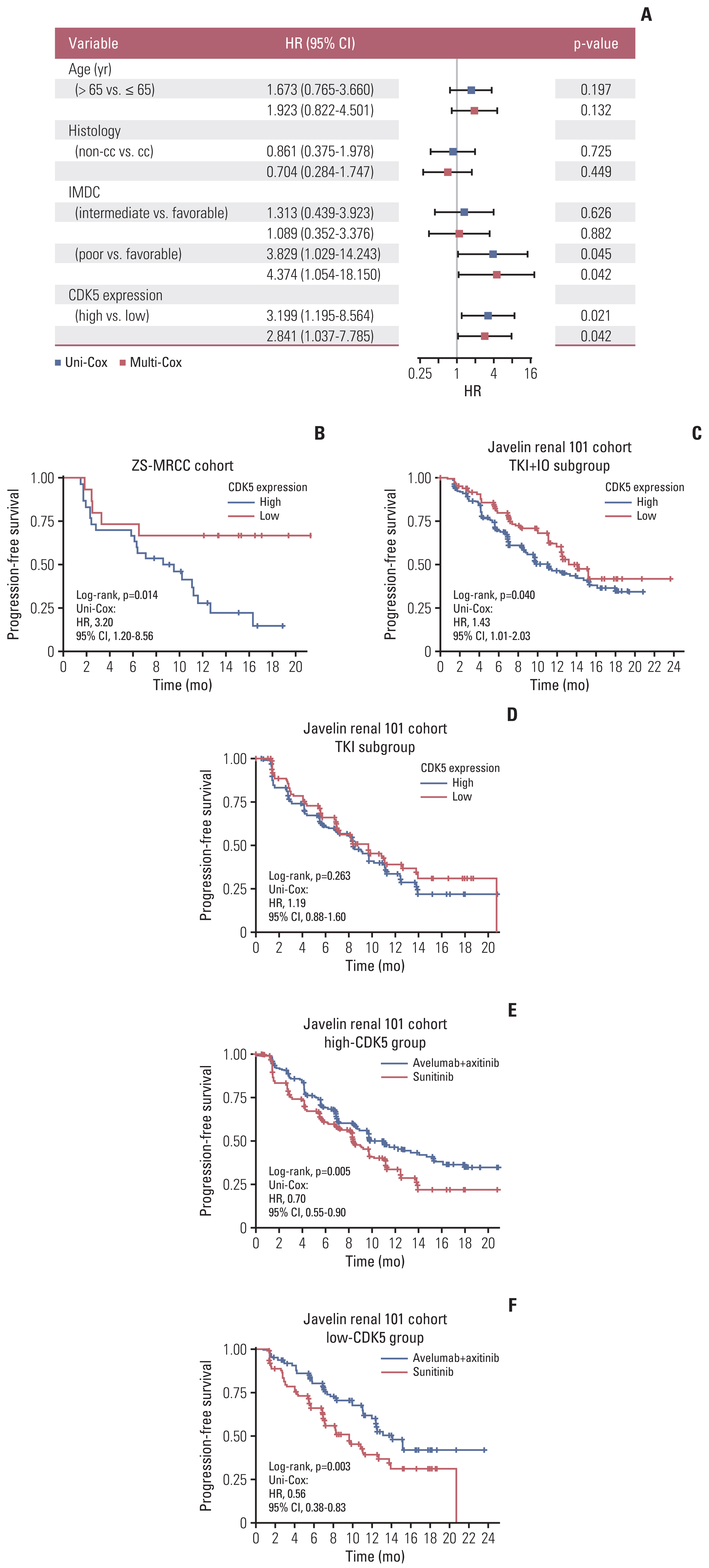
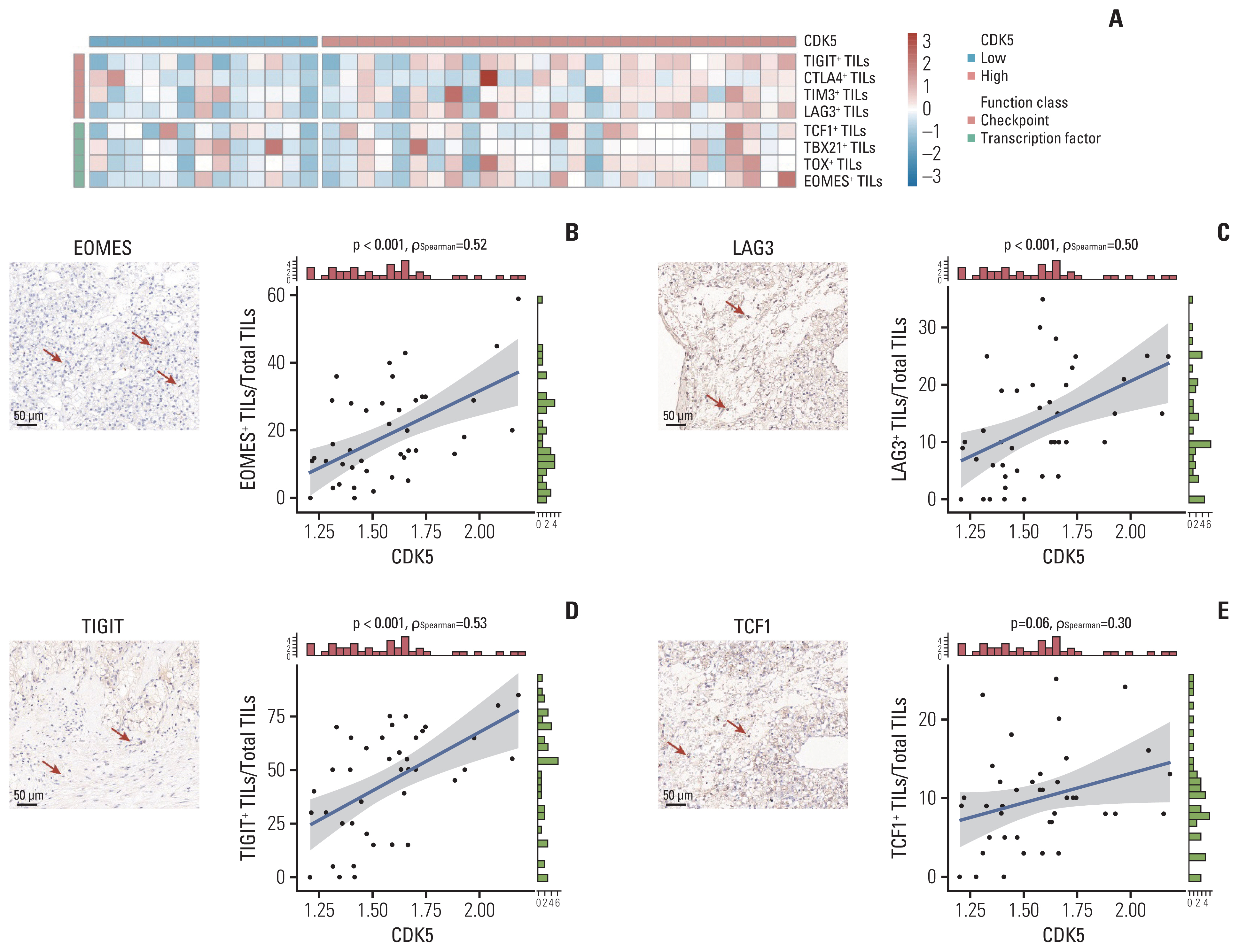
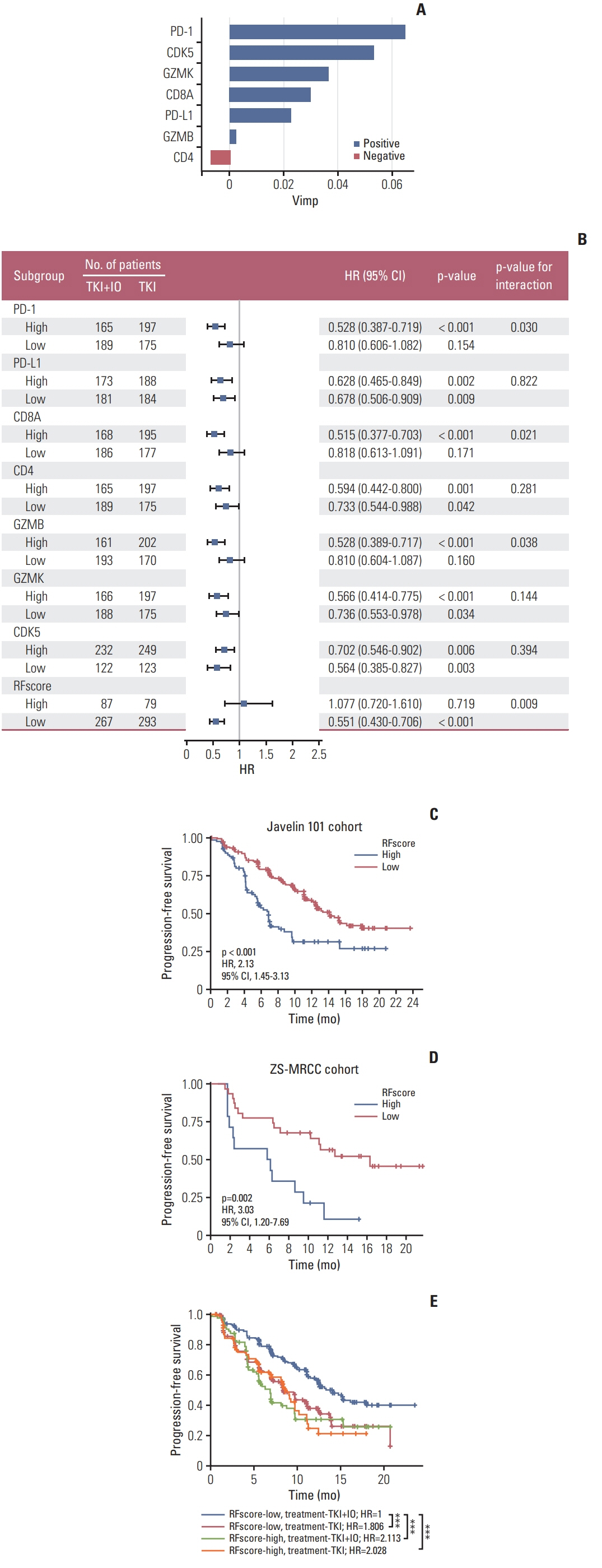
Patients of low CDK5 expression showed higher objective response rate (60.0% vs. 23.3%) and longer PFS in both cohorts (ZS-MRCC cohort, p=0.014; JAVELIN-101 cohort, p=0.040). CDK5 expression was enhanced in non-responders (p < 0.05). In the ZS-HRRCC cohort, CDK5 was associated with decreased tumor-infiltrating CD8+ T cells, which was proved by immunohistochemistry (p < 0.05) and flow cytometry (Spearman’s ρ=–0.49, p < 0.001). In the high CDK5 subgroup, CD8+ T cells revealed a dysfunction phenotype with decreased granzyme B, and more regulatory T cells were identified. A predictive score was further constructed by random forest, involving CDK5 and T cell exhaustion features. The RFscore was also validated in both cohorts. By utilizing the model, more patients might be distinguished from the overall cohort. Additionally, only in the low RFscore did TKI+IO outperform TKI monotherapy.
Conclusion
High-CDK5 expression was associated with immunosuppression and TKI+IO resistance. RFscore based on CDK5 may be utilized as a biomarker to determine the optimal treatment strategy.
Keyword
Figure
Reference
-
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–49.2. Gill DM, Hahn AW, Hale P, Maughan BL. Overview of current and future first-line systemic therapy for metastatic clear cell renal cell carcinoma. Curr Treat Options Oncol. 2018; 19:6.3. Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019; 380:1103–15.4. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019; 380:1116–27.5. Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018; 378:1277–90.6. Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S, et al. European Association of Urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol. 2022; 82:399–410.7. Malumbres M, Harlow E, Hunt T, Hunter T, Lahti JM, Manning G, et al. Cyclin-dependent kinases: a family portrait. Nat Cell Biol. 2009; 11:1275–6.8. Zeng J, Xie S, Liu Y, Shen C, Song X, Zhou GL, et al. CDK5 functions as a tumor promoter in human lung cancer. J Cancer. 2018; 9:3950–61.9. Wang D, Zhou Y, Hua L, Li J, Zhu N, Liu Y. CDK3, CDK5 and CDK8 proteins as prognostic and potential biomarkers in colorectal cancer patients. Int J Gen Med. 2022; 15:2233–45.10. Zhang X, Wang J, Jia Y, Liu T, Wang M, Lv W, et al. CDK5 neutralizes the tumor suppressing effect of BIN1 via mediating phosphorylation of c-MYC at Ser-62 site in NSCLC. Cancer Cell Int. 2019; 19:226.11. Ruiz de Porras V, Bystrup S, Cabrero-de Las Heras S, Musulen E, Palomero L, Alonso MH, et al. Tumor expression of cyclin-dependent kinase 5 (Cdk5) is a prognostic biomarker and predicts outcome of oxaliplatin-treated metastatic colorectal cancer patients. Cancers (Basel). 2019; 11:1540.12. Huang J, Chen P, Liu K, Liu J, Zhou B, Wu R, et al. CDK1/2/5 inhibition overcomes IFNG-mediated adaptive immune resi-stance in pancreatic cancer. Gut. 2021; 70:890–9.13. Dorand RD, Nthale J, Myers JT, Barkauskas DS, Avril S, Chirieleison SM, et al. Cdk5 disruption attenuates tumor PD-L1 expression and promotes antitumor immunity. Science. 2016; 353:399–403.14. Deng H, Tan S, Gao X, Zou C, Xu C, Tu K, et al. Cdk5 knocking out mediated by CRISPR-Cas9 genome editing for PD-L1 attenuation and enhanced antitumor immunity. Acta Pharm Sin B. 2020; 10:358–73.15. De S, Holvey-Bates EG, Mahen K, Willard B, Stark GR. The ubiquitin E3 ligase FBXO22 degrades PD-L1 and sensitizes cancer cells to DNA damage. Proc Natl Acad Sci U S A. 2021; 118:e2112674118.16. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.17. Wang J, Zhang S, Wang Y, Zhu Y, Xu X, Guo J. Alternative complement pathway signature determines immunosuppression and resistance to immunotherapy plus tyrosine kinase inhibitor combinations in renal cell carcinoma. Urol Oncol. 2023; 41:51.18. Xu X, Wang Y, Chen Z, Zhu Y, Wang J, Guo J. Unfavorable immunotherapy plus tyrosine kinase inhibition outcome of metastatic renal cell carcinoma after radical nephrectomy with increased ADAM9 expression. Immunogenetics. 2023; 75:133–43.19. Goldman MJ, Craft B, Hastie M, Repecka K, McDade F, Kamath A, et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat Biotechnol. 2020; 38:675–8.20. Wang J, Liu L, Bai Q, Ou C, Xiong Y, Qu Y, et al. Tumor-infiltrating neutrophils predict therapeutic benefit of tyrosine kinase inhibitors in metastatic renal cell carcinoma. Oncoimmunology. 2019; 8:e1515611.21. Maj T, Wang W, Crespo J, Zhang H, Wang W, Wei S, et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol. 2017; 18:1332–41.22. Zhu L, Ding R, Zhang J, Zhang J, Lin Z. Cyclin-dependent kinase 5 acts as a promising biomarker in clear cell renal cell carcinoma. BMC Cancer. 2019; 19:698.23. Liu JL, Wang XY, Huang BX, Zhu F, Zhang RG, Wu G. Expression of CDK5/p35 in resected patients with non-small cell lung cancer: relation to prognosis. Med Oncol. 2011; 28:673–8.24. Liang Q, Li L, Zhang J, Lei Y, Wang L, Liu DX, et al. CDK5 is essential for TGF-beta1-induced epithelial-mesenchymal transition and breast cancer progression. Sci Rep. 2013; 3:2932.25. Chiker S, Pennaneach V, Loew D, Dingli F, Biard D, Cordelieres FP, et al. Cdk5 promotes DNA replication stress checkpoint activation through RPA-32 phosphorylation, and impacts on metastasis free survival in breast cancer patients. Cell Cycle. 2015; 14:3066–78.26. Catania A, Urban S, Yan E, Hao C, Barron G, Allalunis-Turner J. Expression and localization of cyclin-dependent kinase 5 in apoptotic human glioma cells. Neuro Oncol. 2001; 3:89–98.27. Zhang X, Zhong T, Dang Y, Li Z, Li P, Chen G. Aberrant expression of CDK5 infers poor outcomes for nasopharyngeal carcinoma patients. Int J Clin Exp Pathol. 2015; 8:8066–74.28. Futatsugi A, Utreras E, Rudrabhatla P, Jaffe H, Pant HC, Kulkarni AB. Cyclin-dependent kinase 5 regulates E2F transcription factor through phosphorylation of Rb protein in neurons. Cell Cycle. 2012; 11:1603–10.29. Hsu FN, Chen MC, Lin KC, Peng YT, Li PC, Lin E, et al. Cyclin-dependent kinase 5 modulates STAT3 and androgen receptor activation through phosphorylation of Ser(7)(2)(7) on STAT3 in prostate cancer cells. Am J Physiol Endocrinol Metab. 2013; 305:E975–86.30. Lindqvist J, Imanishi SY, Torvaldson E, Malinen M, Remes M, Orn F, et al. Cyclin-dependent kinase 5 acts as a critical determinant of AKT-dependent proliferation and regulates differential gene expression by the androgen receptor in prostate cancer cells. Mol Biol Cell. 2015; 26:1971–84.31. Prince G, Yang TY, Lin H, Chen MC. Mechanistic insight of cyclin-dependent kinase 5 in modulating lung cancer growth. Chin J Physiol. 2019; 62:231–40.32. Lam E, Choi SH, Pareek TK, Kim BG, Letterio JJ. Cyclin-dependent kinase 5 represses Foxp3 gene expression and Treg development through specific phosphorylation of Stat3 at Serine 727. Mol Immunol. 2015; 67:317–24.33. Lam E, Pareek TK, Letterio JJ. Cdk5 controls IL-2 gene expression via repression of the mSin3a-HDAC complex. Cell Cycle. 2015; 14:1327–36.34. Bei Y, Cheng N, Chen T, Shu Y, Yang Y, Yang N, et al. CDK5 inhibition abrogates TNBC stem-cell property and enhances anti-PD-1 therapy. Adv Sci (Weinh). 2020; 7:2001417.35. Gao L, Xia L, Ji W, Zhang Y, Xia W, Lu S. Knockdown of CDK5 down-regulates PD-L1 via the ubiquitination-proteasome pathway and improves antitumor immunity in lung adenocarcinoma. Transl Oncol. 2021; 14:101148.36. Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016; 16:275–87.37. Chang AL, Miska J, Wainwright DA, Dey M, Rivetta CV, Yu D, et al. CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid-derived suppressor cells. Cancer Res. 2016; 76:5671–82.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Metastatic Renal Cell Carcinoma to the Maxillary Sinus Treated with Tyrosine Kinase Inhibitor
- Developmental mRNA Expression of cdk5 and its Putative Regulators, p67 and p35, in Rat Brain
- Combined Effect of Angioinfarction with Immunotherapy in Patients with Stage IV Renal Cell Carcinoma
- Interferon treatment for Japanese patients with favorable-risk metastatic renal cell carcinoma in the era of targeted therapy
- Perspectives of Targeted Therapies for Sarcomas