J Yeungnam Med Sci.
2023 Oct;40(4):328-334. 10.12701/jyms.2023.00731.
The pathophysiology of diabetic foot: a narrative review
- Affiliations
-
- 1Department of Orthopaedic Surgery, Kosin University College of Medicine, Busan, Korea
- KMID: 2547355
- DOI: http://doi.org/10.12701/jyms.2023.00731
Abstract
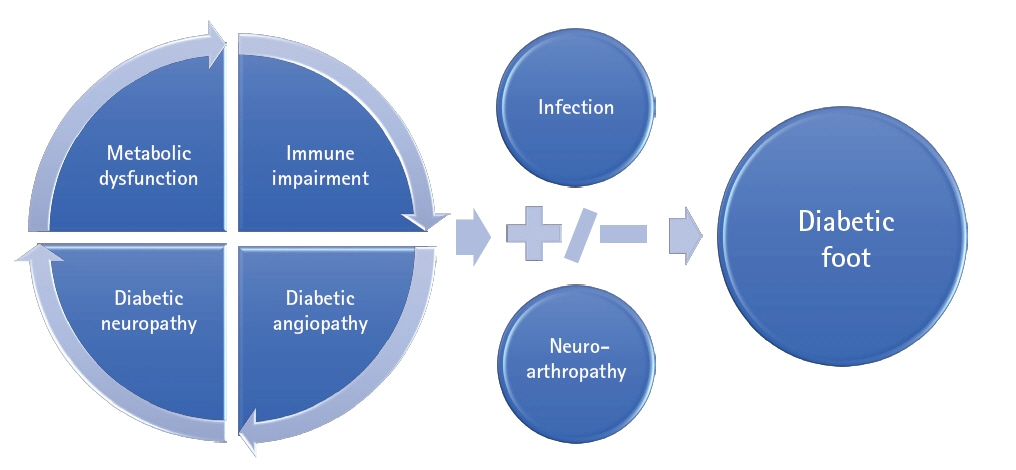
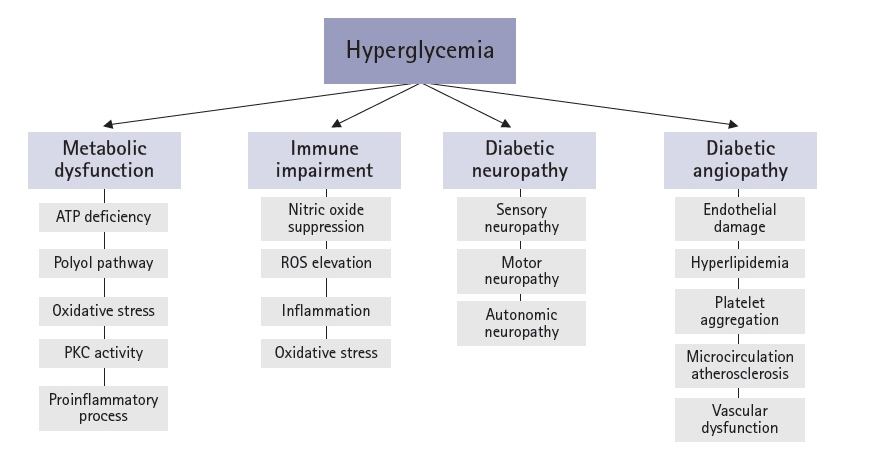
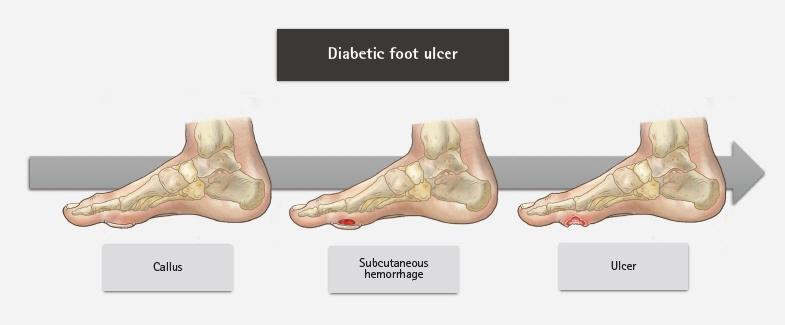
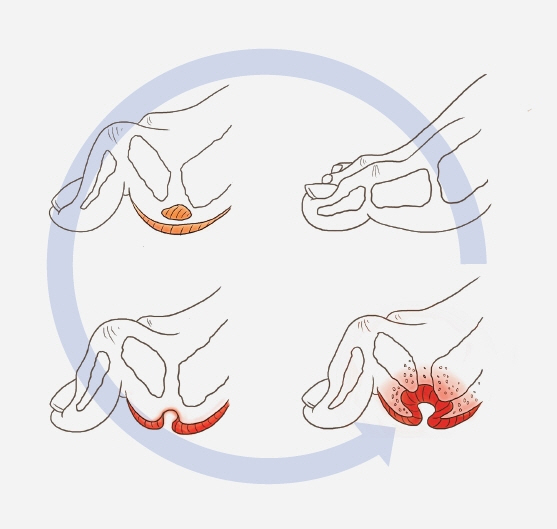
- An aging population and changes in dietary habits have increased the incidence of diabetes, resulting in complications such as diabetic foot ulcers (DFUs). DFUs can lead to serious disabilities, substantial reductions in patient quality of life, and high financial costs for society. By understanding the etiology and pathophysiology of DFUs, their occurrence can be prevented and managed more effectively. The pathophysiology of DFUs involves metabolic dysfunction, diabetic immunopathy, diabetic neuropathy, and angiopathy. The processes by which hyperglycemia causes peripheral nerve damage are related to adenosine triphosphate deficiency, the polyol pathway, oxidative stress, protein kinase C activity, and proinflammatory processes. In the context of hyperglycemia, the suppression of endothelial nitric oxide production leads to microcirculation atherosclerosis, heightened inflammation, and abnormal intimal growth. Diabetic neuropathy involves sensory, motor, and autonomic neuropathies. The interaction between these neuropathies forms a callus that leads to subcutaneous hemorrhage and skin ulcers. Hyperglycemia causes peripheral vascular changes that result in endothelial cell dysfunction and decreased vasodilator secretion, leading to ischemia. The interplay among these four preceding pathophysiological factors fosters the development and progression of infections in individuals with diabetes. Charcot neuroarthropathy is a chronic and progressive degenerative arthropathy characterized by heightened blood flow, increased calcium dissolution, and repeated minor trauma to insensate joints. Directly and comprehensively addressing the pathogenesis of DFUs could pave the way for the development of innovative treatment approaches with the potential to avoid the most serious complications, including major amputations.
Keyword
Figure
Cited by 1 articles
-
Unveiling the challenges of diabetic foot infections: diagnosis, pathogenesis, treatment, and rehabilitation
Chul Hyun Park
J Yeungnam Med Sci. 2023;40(4):319-320. doi: 10.12701/jyms.2023.01011.
Reference
-
References
1. Sumpio BE. Contemporary evaluation and management of the diabetic foot. Scientifica (Cairo). 2012; 2012:435487.
Article2. Boulton AJ. Hyperbaric oxygen in the management of chronic diabetic foot ulcers. Curr Diab Rep. 2010; 10:255–6.
Article3. Boulton AJ, Armstrong DG. Diabetic foot and ulceration: epidemiology and pathophysiology. In : Falabella A, Kirsner R, editors. Wound healing. Boca Raton: CRC Press:;2005.4. Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, et al. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A. 2000; 97:12222–6.
Article5. Grover-Johnson NM, Baumann FG, Imparato AM, Kim GE, Thomas PK. Abnormal innervation of lower limb epineurial arterioles in human diabetes. Diabetologia. 1981; 20:31–8.
Article6. Yagihashi S, Mizukami H, Sugimoto K. Mechanism of diabetic neuropathy: where are we now and where to go? J Diabetes Investig. 2011; 2:18–32.
Article7. Fernyhough P, McGavock J. Mechanisms of disease: mitochondrial dysfunction in sensory neuropathy and other complications in diabetes. Handb Clin Neurol. 2014; 126:353–77.8. Singh VP, Bali A, Singh N, Jaggi AS. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol. 2014; 18:1–14.
Article9. Ollendorf DA, Kotsanos JG, Wishner WJ, Friedman M, Cooper T, Bittoni M, et al. Potential economic benefits of lower-extremity amputation prevention strategies in diabetes. Diabetes Care. 1998; 21:1240–5.
Article10. Yagihashi S, Yamagishi SI, Mizukami H, Ogasawara S, Chung S, Chung SK. Escape phenomenon from polyol pathway to other metabolic cascades may underlie nerve conduction delay in severely hyperglycemic AR-deficient mice (abstract No. 775-P). Abstract presented at: American Diabetes Association, 68th Scientific Sessions. 2008 June 6-10; San Francisco. American Diabetes Association;2008.11. Mochly-Rosen D, Das K, Grimes KV. Protein kinase C, an elusive therapeutic target? Nat Rev Drug Discov. 2012; 11:937–57.
Article12. Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992; 258:607–14.
Article13. Wang QJ. PKD at the crossroads of DAG and PKC signaling. Trends Pharmacol Sci. 2006; 27:317–23.
Article14. Schambelan M, Blake S, Sraer J, Bens M, Nivez MP, Wahbe F. Increased prostaglandin production by glomeruli isolated from rats with streptozotocin-induced diabetes mellitus. J Clin Invest. 1985; 75:404–12.15. Gutiérrez A, Contreras C, Sánchez A, Prieto D. Role of phosphatidylinositol 3-kinase (PI3K), mitogen-activated protein kinase (MAPK), and protein kinase C (PKC) in calcium signaling pathways linked to the α1-adrenoceptor in resistance arteries. Front Physiol. 2019; 10:55.
Article16. Retamal JS, Grace MS, Dill LK, Ramirez-Garcia P, Peng S, Gondin AB, et al. Serotonin-induced vascular permeability is mediated by transient receptor potential vanilloid 4 in the airways and upper gastrointestinal tract of mice. Lab Invest. 2021; 101:851–64.
Article17. Moriya J, Ferrara N. Inhibition of protein kinase C enhances angiogenesis induced by platelet-derived growth factor C in hyperglycemic endothelial cells. Cardiovasc Diabetol. 2015; 14:19.18. Baum P, Toyka KV, Blüher M, Kosacka J, Nowicki M. Inflammatory mechanisms in the pathophysiology of diabetic peripheral neuropathy (DN): new aspects. Int J Mol Sci. 2021; 22:10835.
Article19. Eslami MH, Zayaruzny M, Fitzgerald GA. The adverse effects of race, insurance status, and low income on the rate of amputation in patients presenting with lower extremity ischemia. J Vasc Surg. 2007; 45:55–9.
Article20. Yao D, Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes. 2010; 59:249–55.
Article21. Wu H, Cai L, de Haan JB, Giacconi R. Targeting oxidative stress in diabetic complications: new insights. J Diabetes Res. 2018; 2018:1909675.
Article22. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010; 107:1058–70.
Article23. Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005; 28:956–62.24. Strotmeyer ES, de Rekeneire N, Schwartz AV, Resnick HE, Goodpaster BH, Faulkner KA, et al. Sensory and motor peripheral nerve function and lower-extremity quadriceps strength: the health, aging and body composition study. J Am Geriatr Soc. 2009; 57:2004–10.
Article25. Clayton W, Elasy TA. A review of the pathophysiology, classification, and treatment of foot ulcers in diabetic patients. Clin Diabetes. 2009; 27:52–8.
Article26. Sabanayagam C, Liew G, Tai ES, Shankar A, Lim SC, Subramaniam T, et al. Relationship between glycated haemoglobin and microvascular complications: is there a natural cut-off point for the diagnosis of diabetes? Diabetologia. 2009; 52:1279–89.
Article27. Pop-Busui R, Lu J, Lopes N, Jones TL; BARI 2D Investigators. Prevalence of diabetic peripheral neuropathy and relation to glycemic control therapies at baseline in the BARI 2D cohort. J Peripher Nerv Syst. 2009; 14:1–13.
Article28. Kihara M, Weerasuriya A, Low PA. Endoneurial blood flow in rat sciatic nerve during development. J Physiol. 1991; 439:351–60.
Article29. Dickenson AH, Matthews EA, Suzuki R. Neurobiology of neuropathic pain: mode of action of anticonvulsants. Eur J Pain. 2002; 6(Suppl A):51–60.
Article30. Black JA, Cummins TR, Plumpton C, Chen YH, Hormuzdiar W, Clare JJ, et al. Upregulation of a silent sodium channel after peripheral, but not central, nerve injury in DRG neurons. J Neurophysiol. 1999; 82:2776–85.
Article31. He XH, Zang Y, Chen X, Pang RP, Xu JT, Zhou X, et al. TNF-α contributes to up-regulation of Nav1.3 and Nav1.8 in DRG neurons following motor fiber injury. Pain. 2010; 151:266–79.32. Kim J, Wei DS, Hoffman DA. Kv4 potassium channel subunits control action potential repolarization and frequency-dependent broadening in rat hippocampal CA1 pyramidal neurones. J Physiol. 2005; 569:41–57.33. Feldman EL, Russell JW, Sullivan KA, Golovoy D. New insights into the pathogenesis of diabetic neuropathy. Curr Opin Neurol. 1999; 12:553–63.
Article34. Zochodne DW. Diabetic polyneuropathy: an update. Curr Opin Neurol. 2008; 21:527–33.
Article35. Boulton AJ, Kirsner RS, Vileikyte L. Clinical practice. Neuropathic diabetic foot ulcers. N Engl J Med. 2004; 351:48–55.36. Russell JW, Zilliox LA. Diabetic neuropathies. Continuum (Minneap Minn). 2014; 20:1226–40.
Article37. Volmer-Thole M, Lobmann R. Neuropathy and diabetic foot syndrome. Int J Mol Sci. 2016; 17:917.38. Trieb K. The Charcot foot: pathophysiology, diagnosis and classification. Bone Joint J. 2016; 98-B:1155–9.39. Bansal V, Kalita J, Misra UK. Diabetic neuropathy. Postgrad Med J. 2006; 82:95–100.40. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017; 376:2367–75.
Article41. Paraskevas KI, Baker DM, Pompella A, Mikhailidis DP. Does diabetes mellitus play a role in restenosis and patency rates following lower extremity peripheral arterial revascularization? A critical overview. Ann Vasc Surg. 2008; 22:481–91.
Article42. Janka HU, Standl E, Mehnert H. Peripheral vascular disease in diabetes mellitus and its relation to cardiovascular risk factors: screening with the doppler ultrasonic technique. Diabetes Care. 1980; 3:207–13.
Article43. Williams DT, Hilton JR, Harding KG. Diagnosing foot infection in diabetes. Clin Infect Dis. 2004; 39(Suppl 2):S83–6.
Article44. Yamagishi SI, Edelstein D, Du XL, Brownlee M. Hyperglycemia potentiates collagen-induced platelet activation through mitochondrial superoxide overproduction. Diabetes. 2001; 50:1491–4.
Article45. Mamputu JC, Renier G. Advanced glycation end products increase, through a protein kinase C-dependent pathway, vascular endothelial growth factor expression in retinal endothelial cells. Inhibitory effect of gliclazide. J Diabetes Complications. 2002; 16:284–93.46. Bandyk DF. The diabetic foot: pathophysiology, evaluation, and treatment. Semin Vasc Surg. 2018; 31:43–8.
Article47. Schaper NC, Van Netten JJ, Apelqvist J, Lipsky BA, Bakker K; International Working Group on the Diabetic Foot. Prevention and management of foot problems in diabetes: a Summary Guidance for Daily Practice 2015, based on the IWGDF Guidance Documents. Diabetes Metab Res Rev. 2016; 32(Suppl 1):7–15.
Article48. Stacey HJ, Clements CS, Welburn SC, Jones JD. The prevalence of methicillin-resistant Staphylococcus aureus among diabetic patients: a meta-analysis. Acta Diabetol. 2019; 56:907–21.
Article49. Saltoglu N, Ergonul O, Tulek N, Yemisen M, Kadanali A, Karagoz G, et al. Influence of multidrug resistant organisms on the outcome of diabetic foot infection. Int J Infect Dis. 2018; 70:10–4.
Article50. Icks A, Scheer M, Morbach S, Genz J, Haastert B, Giani G, et al. Time-dependent impact of diabetes on mortality in patients after major lower extremity amputation: survival in a population-based 5-year cohort in Germany. Diabetes Care. 2011; 34:1350–4.51. Kerr M; Insight Health Economics. Foot care for people with diabetes: the economic case for change. National Health Service;UK: 2012.52. Singh S, Pai DR, Yuhhui C. Diabetic foot ulcer-diagnosis and management. Clin Res Foot Ankle. 2013; 1:120.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- State-of-the-art update for diagnosing diabetic foot osteomyelitis: a narrative review
- Management of diabetic foot ulcers: a narrative review
- Diagnosis and Management of Diabetic Foot
- Management and rehabilitation of moderate-to-severe diabetic foot infection: a narrative review
- Prevention and Education for Diabetic Foot