Nutr Res Pract.
2023 Oct;17(5):917-933. 10.4162/nrp.2023.17.5.917.
Peanut sprout tea extract inhibits lung metastasis of 4T1 murine mammary carcinoma cells by suppressing the crosstalk between cancer cells and macrophages in BALB/c mice
- Affiliations
-
- 1Industry Coupled Cooperation Center for Bio Healthcare Materials, Hallym University, Chuncheon 24252, Korea
- 2Department of Food Science & Nutrition, Dongseo University, Busan 47011, Korea
- 3Department of Applied Animal Science, Kangwon National University, Chuncheon 24341, Korea
- KMID: 2546948
- DOI: http://doi.org/10.4162/nrp.2023.17.5.917
Abstract
- BACKGROUND/OBJECTIVES
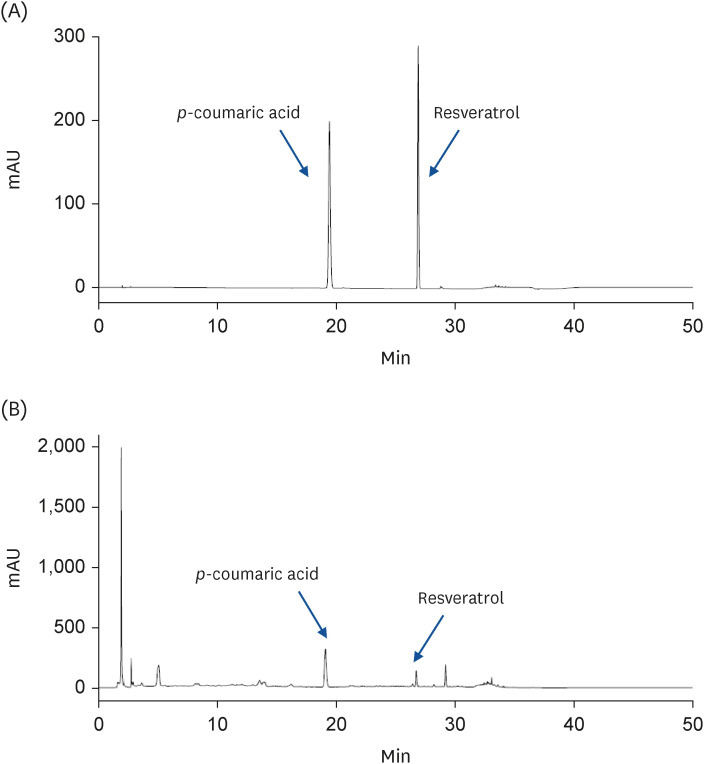
As peanuts germinate, the content of the components beneficial to health, such as resveratrol, increases within the peanut sprout. This study examined whether the ethanol extract of peanut sprout tea (PSTE) inhibits breast cancer growth and metastasis.
MATERIALS/METHODS
After orthotopically injecting 4T1 cells into BALB/c mice to induce breast cancer, 0, 30, or 60 mg/kg body weight/day of PSTE was administered orally. Angiogenesis-related protein expression in the tumors and the degree of metastasis were analyzed. 4T1 and RAW 264.7 cells were co-cultured, and reverse transcription polymerase chain reaction was performed to measure the crosstalk between breast cancer cells and macrophages.
RESULTS
PSTE reduced tumor growth and lung metastasis. In particular, PSTE decreased matrix metalloproteinase-9, platelet endothelial cell adhesion molecule-1, vascular endothelial growth factor-A, F4/80, CD11c, macrophage mannose receptor, macrophage colony-stimulating factor, and monocyte chemoattractant protein 1 expression in the tumors. Moreover, PSTE prevented 4T1 cell migration, invasion, and macrophage activity in RAW 264.7 cells. PSTE inhibited the crosstalk between 4T1 cells and RAW 264.7 cells and promoted the macrophage M1 subtype while inhibiting the M2 subtype.
CONCLUSIONS
These results suggest that PSTE blocks breast cancer growth and metastasis to the lungs. This may be because the PSTE treatment inhibits the crosstalk between mammary cancer cells and macrophages and inhibits the differentiation of macrophages into the M2 subtype.
Keyword
Figure
Reference
-
1. Villarini M, Lanari C, Nucci D, Gianfredi V, Marzulli T, Berrino F, Borgo A, Bruno E, Gargano G, Moretti M, et al. Community-based participatory research to improve life quality and clinical outcomes of patients with breast cancer (DianaWeb in Umbria pilot study). BMJ Open. 2016; 6:e009707.2. Ghoncheh M, Pournamdar Z, Salehiniya H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac J Cancer Prev. 2016; 17:43–46.3. Spano D, Heck C, De Antonellis P, Christofori G, Zollo M. Molecular networks that regulate cancer metastasis. Semin Cancer Biol. 2012; 22:234–249. PMID: 22484561.4. Chiang AC, Massagué J. Molecular basis of metastasis. N Engl J Med. 2008; 359:2814–2823. PMID: 19109576.5. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. Molecular portraits of human breast tumours. Nature. 2000; 406:747–752. PMID: 10963602.6. Xiao W, Zheng S, Liu P, Zou Y, Xie X, Yu P, Tang H, Xie X. Risk factors and survival outcomes in patients with breast cancer and lung metastasis: a population-based study. Cancer Med. 2018; 7:922–930. PMID: 29473333.7. Jin X, Mu P. Targeting breast cancer metastasis. Breast Cancer (Auckl). 2015; 9:23–34. PMID: 26380552.8. Montané X, Kowalczyk O, Reig-Vano B, Bajek A, Roszkowski K, Tomczyk R, Pawliszak W, Giamberini M, Mocek-Płóciniak A, Tylkowski B. Current perspectives of the applications of polyphenols and flavonoids in cancer therapy. Molecules. 2020; 25:3342. PMID: 32717865.9. Gan RY, Wang MF, Lui WY, Wu K, Corke H. Dynamic changes in phytochemical composition and antioxidant capacity in green and black mung bean (Vigna radiata) sprouts. Int J Food Sci Technol. 2016; 51:2090–2098.10. Lazo-Vélez MA, Guardado-Félix D, Avilés-González J, Romo-López I, Serna-Saldívar SO. Effect of germination with sodium selenite on the isoflavones and cellular antioxidant activity of soybean (Glycine max). Lebensm Wiss Technol. 2018; 93:64–70.11. Yang QQ, Cheng L, Long ZY, Li HB, Gunaratne A, Gan RY, Corke H. Comparison of the phenolic profiles of soaked and germinated peanut cultivars via UPLC-QTOF-MS. Antioxidants (Basel). 2019; 8:47. PMID: 30791635.12. Wang KH, Lai YH, Chang JC, Ko TF, Shyu SL, Chiou RY. Germination of peanut kernels to enhance resveratrol biosynthesis and prepare sprouts as a functional vegetable. J Agric Food Chem. 2005; 53:242–246. PMID: 15656656.13. Langcake P, Pryce RJ. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol Plant Pathol. 1976; 9:77–86.14. Gan RY, Xu XR, Song FL, Kuang L, Li HB. Antioxidant activity and total phenolic content of medicinal plants associated with prevention and treatment of cardiovascular and cerebrovascular diseases. J Med Plants Res. 2010; 4:2438–2444.15. Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004; 117:927–939. PMID: 15210113.16. Fantozzi A, Christofori G. Mouse models of breast cancer metastasis. Breast Cancer Res. 2006; 8:212. PMID: 16887003.17. Kim EJ, Holthuizen PE, Park HS, Ha YL, Jung KC, Park JH. Trans-10,cis-12-conjugated linoleic acid inhibits Caco-2 colon cancer cell growth. Am J Physiol Gastrointest Liver Physiol. 2002; 283:G357–G367. PMID: 12121883.18. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016; 7:27–31. PMID: 27057123.19. Sauter BV, Martinet O, Zhang WJ, Mandeli J, Woo SL. Adenovirus-mediated gene transfer of endostatin in vivo results in high level of transgene expression and inhibition of tumor growth and metastases. Proc Natl Acad Sci U S A. 2000; 97:4802–4807. PMID: 10758166.20. Cho HJ, Jung JI, Lim DY, Kwon GT, Her S, Park JH, Park JH. Bone marrow-derived, alternatively activated macrophages enhance solid tumor growth and lung metastasis of mammary carcinoma cells in a Balb/C mouse orthotopic model. Breast Cancer Res. 2012; 14:R81. PMID: 22616919.21. Lee HS, Jung JI, Kim KH, Park SJ, Kim EJ. Rhus verniciflua stokes extract suppresses migration and invasion in human gastric adenocarcinoma AGS cells. Nutr Res Pract. 2020; 14:463–477. PMID: 33029287.22. Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008; 66:1–9. PMID: 17913510.23. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002; 23:549–555. PMID: 12401408.24. Panni RZ, Linehan DC, DeNardo DG. Targeting tumor-infiltrating macrophages to combat cancer. Immunotherapy. 2013; 5:1075–1087. PMID: 24088077.25. Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997; 275:218–220. PMID: 8985016.26. Delmas D, Jannin B, Cherkaoui Malki M, Latruffe N. Inhibitory effect of resveratrol on the proliferation of human and rat hepatic derived cell lines. Oncol Rep. 2000; 7:847–852. PMID: 10854556.27. Delmas D, Passilly-Degrace P, Jannin B, Cherkaoui Malki M, Latruffe N. Resveratrol, a chemopreventive agent, disrupts the cell cycle control of human SW480 colorectal tumor cells. Int J Mol Med. 2002; 10:193–199. PMID: 12119558.28. Frazzi R, Valli R, Tamagnini I, Casali B, Latruffe N, Merli F. Resveratrol-mediated apoptosis of hodgkin lymphoma cells involves SIRT1 inhibition and FOXO3a hyperacetylation. Int J Cancer. 2013; 132:1013–1021. PMID: 22833338.29. Singh SK, Banerjee S, Acosta EP, Lillard JW, Singh R. Resveratrol induces cell cycle arrest and apoptosis with docetaxel in prostate cancer cells via a p53/ p21WAF1/CIP1 and p27KIP1 pathway. Oncotarget. 2017; 8:17216–17228. PMID: 28212547.30. Karatay KB, Yurt Kilcar A, Kozgus Guldu O, Medine EI, Biber Muftuler FZ. Isolation of resveratrol from peanut sprouts, radioiodination and investigation of its bioactivity on neuroblastoma cell lines. J Radioanal Nucl Chem. 2020; 325:75–84.31. Park H, Song JH, Hwang B, Moon B, Yun SJ, Kim WJ, Moon SK. Evaluation of the in vitro and in vivo antitumor efficacy of peanut sprout extracts cultivated with fermented sawdust medium against bladder cancer. Appl Sci. 2020; 10:8758.32. Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli G, et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010; 70:9003–9011. PMID: 20935227.33. Howells LM, Berry DP, Elliott PJ, Jacobson EW, Hoffmann E, Hegarty B, Brown K, Steward WP, Gescher AJ. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases--safety, pharmacokinetics, and pharmacodynamics. Cancer Prev Res (Phila). 2011; 4:1419–1425. PMID: 21680702.34. Jaganathan SK, Supriyanto E, Mandal M. Events associated with apoptotic effect of p-coumaric acid in HCT-15 colon cancer cells. World J Gastroenterol. 2013; 19:7726–7734. PMID: 24282361.35. Jang MG, Ko HC, Kim SJ. Effects of p-coumaric acid on microRNA expression profiles in SNU-16 human gastric cancer cells. Genes Genomics. 2020; 42:817–825. PMID: 32462517.36. Vervandier-Fasseur D, Latruffe N. The potential use of resveratrol for cancer prevention. Molecules. 2019; 24:4506. PMID: 31835371.37. Colin D, Gimazane A, Lizard G, Izard JC, Solary E, Latruffe N, Delmas D. Effects of resveratrol analogs on cell cycle progression, cell cycle associated proteins and 5fluoro-uracil sensitivity in human derived colon cancer cells. Int J Cancer. 2009; 124:2780–2788. PMID: 19334045.38. Latruffe N, Rifler JP. Bioactive polyphenols from grapes and wine emphasized with resveratrol. Curr Pharm Des. 2013; 19:6053–6063. PMID: 23448444.39. Hunter KW, Crawford NP, Alsarraj J. Mechanisms of metastasis. Breast Cancer Res. 2008; 10(Suppl 1):S2.40. Azevedo AS, Follain G, Patthabhiraman S, Harlepp S, Goetz JG. Metastasis of circulating tumor cells: favorable soil or suitable biomechanics, or both? Cell Adhes Migr. 2015; 9:345–356.41. Polyak K, Kalluri R. The role of the microenvironment in mammary gland development and cancer. Cold Spring Harb Perspect Biol. 2010; 2:a003244. PMID: 20591988.42. Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020; 877:173090. PMID: 32234529.43. Ding Y, Song N, Luo Y. Role of bone marrow-derived cells in angiogenesis: focus on macrophages and pericytes. Cancer Microenviron. 2012; 5:225–236. PMID: 22528877.44. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014; 6:13. PMID: 24669294.45. Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014; 41:14–20. PMID: 25035950.46. Vianello E, Dozio E, Arnaboldi F, Marazzi MG, Martinelli C, Lamont J, Tacchini L, Sigrüner A, Schmitz G, Corsi Romanelli MM. Epicardial adipocyte hypertrophy: association with M1-polarization and toll-like receptor pathways in coronary artery disease patients. Nutr Metab Cardiovasc Dis. 2016; 26:246–253. PMID: 26841679.47. Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol. 2014; 14:81–93. PMID: 24445666.48. Solinas G, Schiarea S, Liguori M, Fabbri M, Pesce S, Zammataro L, Pasqualini F, Nebuloni M, Chiabrando C, Mantovani A, et al. Tumor-conditioned macrophages secrete migration-stimulating factor: a new marker for M2-polarization, influencing tumor cell motility. J Immunol. 2010; 185:642–652. PMID: 20530259.49. Mantovani A, Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med. 2015; 212:435–445. PMID: 25753580.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of resveratrol on the metastasis of 4T1 mouse breast cancer cells in vitro and in vivo
- α-Tocopheryl Succinate Inhibits Osteolytic Bone Metastasis of Breast Cancer by Suppressing Migration of Cancer Cells and Receptor Activator of Nuclear Factor-κB Ligand Expression of Osteoblasts
- Resveratrol from Peanut Sprout Extract Promotes NK Cell Activation and Antitumor Activity
- Immunological Activities of Korean Mistletoe Extract ( Viscum album coloratum ; KM - 110 )
- OCT4 Expression Enhances Features of Cancer Stem Cells in a Mouse Model of Breast Cancer