J Bone Metab.
2018 Feb;25(1):23-33. 10.11005/jbm.2018.25.1.23.
α-Tocopheryl Succinate Inhibits Osteolytic Bone Metastasis of Breast Cancer by Suppressing Migration of Cancer Cells and Receptor Activator of Nuclear Factor-κB Ligand Expression of Osteoblasts
- Affiliations
-
- 1Department of Cell and Developmental Biology, Dental Research Institute, School of Dentistry, Seoul National University College of Medicine, Seoul, Korea. zang1959@snu.ac.kr
- KMID: 2406236
- DOI: http://doi.org/10.11005/jbm.2018.25.1.23
Abstract
- BACKGROUND
Breast cancer is one of the most common cancers affecting women and has a high incidence of bone metastasis, causing osteolytic lesions. The elevated expression of receptor activator of nuclear factor-κB ligand (RANKL) in cancer activates osteoclasts, leading to bone destruction. We previously reported that α-tocopheryl succinate (αTP-suc) inhibited interleukin-1-induced RANKL expression in osteoblasts. Here, we examined the effect of αTP-suc on osteolytic bone metastasis in breast cancer.
METHODS
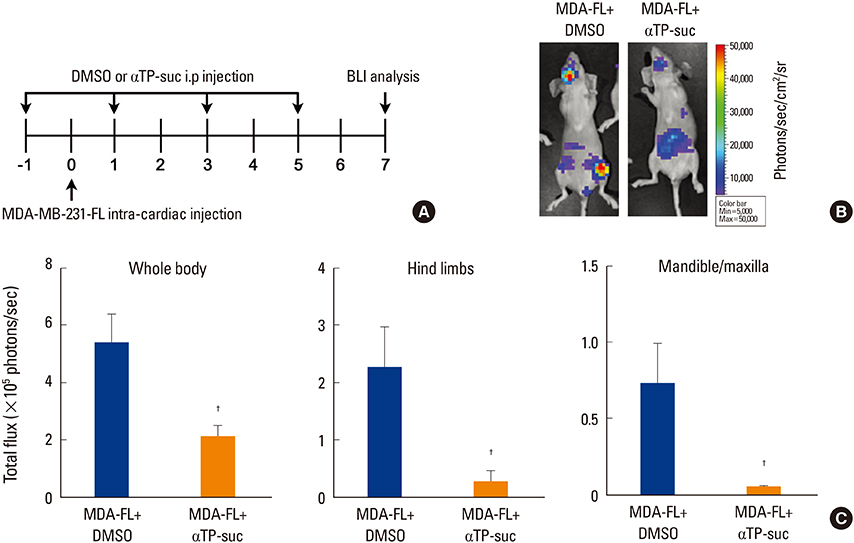
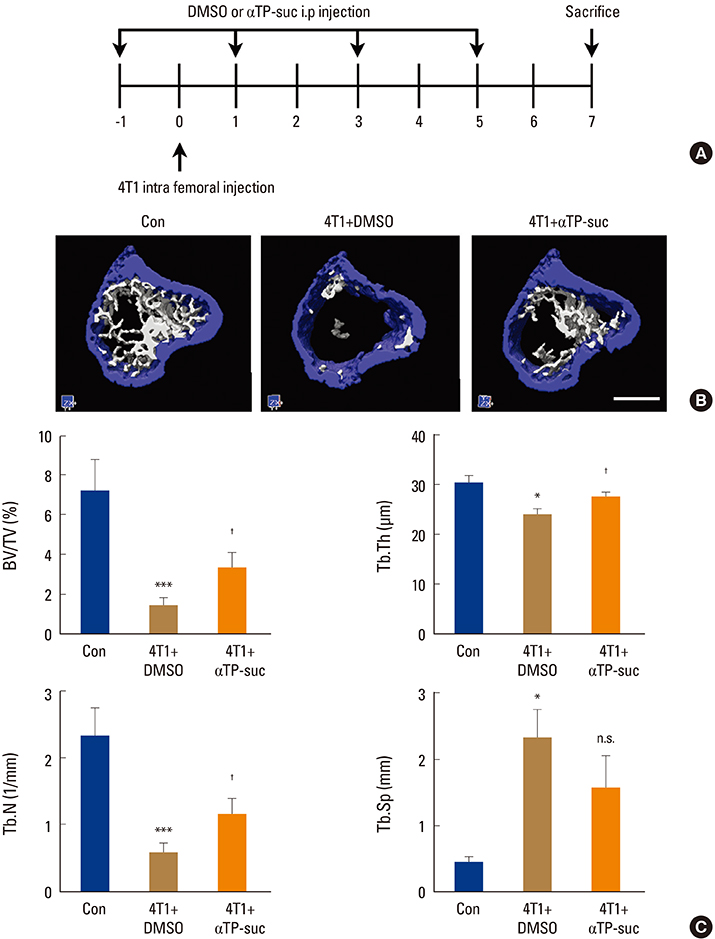
To examine the effect of αTP-suc on the metastatic capacity of breast cancer, MDA-MB-231-FL cells were injected into the left cardiac ventricle of BALB/c nude mice along with intraperitoneal injection of αTP-suc. The mice were then analyzed by bioluminescence imaging. To investigate the effect of αTP-suc on osteolysis, 4T1 cells were directly injected into the femur of BALB/c mice along with intraperitoneal injection of αTP-suc. Microcomputed tomography analysis and histomorphometric analysis of the femora were performed.
RESULTS
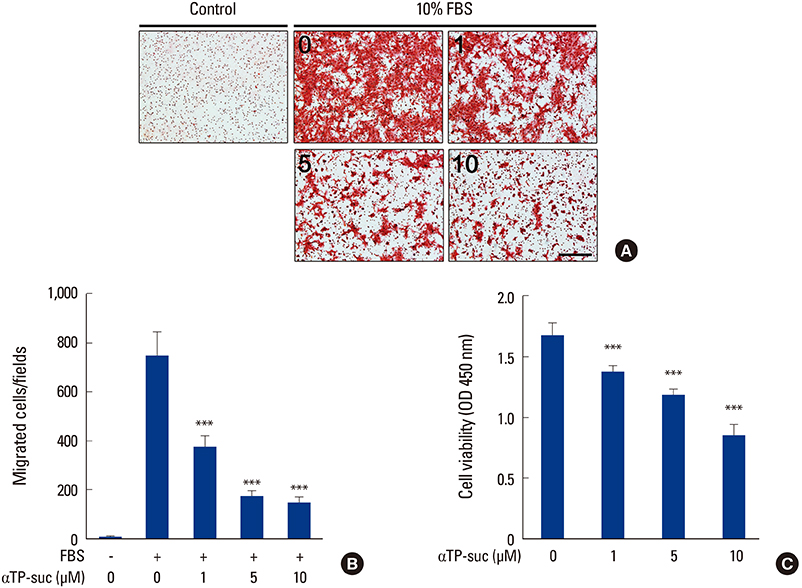
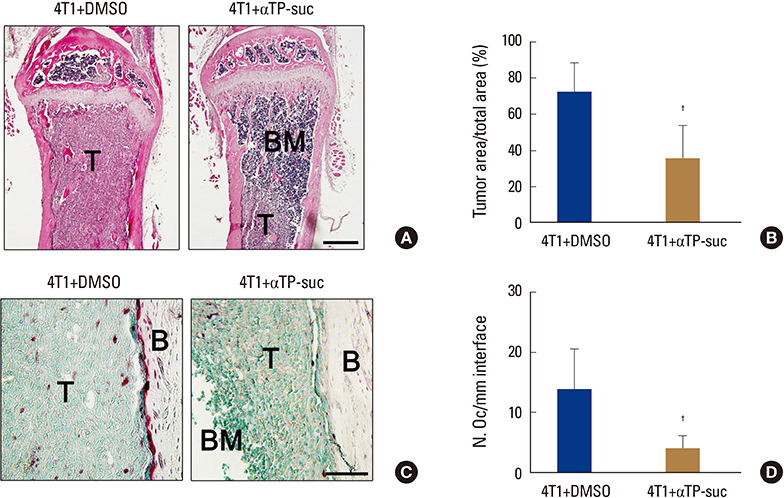
αTP-suc inhibited cell migration and cell growth of 4T1 cells. In line with these results, bone metastasis of MDA-MB-231-FL cells was reduced in mice injected with αTP-suc. In addition, αTP-suc decreased osteoclastogenesis by inhibiting 4T1-induced RANKL expression in osteoblasts. Consistent with these results, 4T1-induced bone destruction was ameliorated by αTP-suc, with in vivo analysis showing reduced tumor burden and osteoclast numbers.
CONCLUSIONS
Our findings suggest that αTP-suc may be efficiently utilized to prevent and treat osteolytic bone metastasis of breast cancer with dual effects.
MeSH Terms
Figure
Cited by 2 articles
-
Causal Inference Network of Genes Related with Bone Metastasis of Breast Cancer and Osteoblasts Using Causal Bayesian Networks
Sung Bae Park, Chun Kee Chung, Efrain Gonzalez, Changwon Yoo
J Bone Metab. 2018;25(4):251-266. doi: 10.11005/jbm.2018.25.4.251.Extracts of Flavoparmelia sp. Inhibit Receptor Activator of Nuclear Factor-κB Ligand-Mediated Osteoclast Differentiation
Kwang-Jin Kim, Yongjin Lee, Min-Hye Jeong, Jae-Seoun Hur, Young-Jin Son
J Bone Metab. 2019;26(2):113-121. doi: 10.11005/jbm.2019.26.2.113.
Reference
-
1. Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004; 350:1655–1664.
Article2. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001; 27:165–176.
Article3. Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002; 2:584–593.
Article4. Chen YC, Sosnoski DM, Mastro AM. Breast cancer metastasis to the bone: mechanisms of bone loss. Breast Cancer Res. 2010; 12:215.
Article5. Wang N, Docherty FE, Brown HK, et al. Prostate cancer cells preferentially home to osteoblast-rich areas in the early stages of bone metastasis: evidence from in vivo models. J Bone Miner Res. 2014; 29:2688–2696.
Article6. Croucher PI, McDonald MM, Martin TJ. Bone metastasis: the importance of the neighbourhood. Nat Rev Cancer. 2016; 16:373–386.
Article7. Ju J, Picinich SC, Yang Z, et al. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis. 2010; 31:533–542.
Article8. Husain K, Francois RA, Yamauchi T, et al. Vitamin E delta-to-cotrienol augments the antitumor activity of gemcitabine and suppresses constitutive NF-kappaB activation in pancreatic cancer. Mol Cancer Ther. 2011; 10:2363–2372.
Article9. Gysin R, Azzi A, Visarius T. Gamma-tocopherol inhibits human cancer cell cycle progression and cell proliferation by down-regulation of cyclins. FASEB J. 2002; 16:1952–1954.
Article10. Alqahtani S, Kaddoumi A. Vitamin E transporters in cancer therapy. AAPS J. 2015; 17:313–322.
Article11. Kim HN, Lee JH, Jin WJ, et al. alpha-Tocopheryl succinate inhibits osteoclast formation by suppressing receptor activator of nuclear factor-kappaB ligand (RANKL) expression and bone resorption. J Bone Metab. 2012; 19:111–120.
Article12. Savitskaya MA, Onischenko GE. alpha-Tocopheryl succinate affects malignant cell viability, proliferation, and differentiation. Biochemistry (Mosc). 2016; 81:806–818.
Article13. Lee JH, Kim HN, Yang D, et al. Trolox prevents osteoclastogenesis by suppressing RANKL expression and signaling. J Biol Chem. 2009; 284:13725–13734.
Article14. Lee JH, Kim HN, Kim KO, et al. CXCL10 promotes osteolytic bone metastasis by enhancing cancer outgrowth and osteoclastogenesis. Cancer Res. 2012; 72:3175–3186.
Article15. Jin WJ, Kim B, Kim D, et al. NF-kappaB signaling regulates cell-autonomous regulation of CXCL10 in breast cancer 4T1 cells. Exp Mol Med. 2017; 49:e295.16. Wang D, Chuang HC, Weng SC, et al. alpha-Tocopheryl succinate as a scaffold to develop potent inhibitors of breast cancer cell adhesion. J Med Chem. 2009; 52:5642–5648.
Article17. Davis-Yadley AH, Malafa MP. Vitamins in pancreatic cancer: a review of underlying mechanisms and future applications. Adv Nutr. 2015; 6:774–802.
Article18. Doldo E, Costanza G, Agostinelli S, et al. Vitamin A, cancer treatment and prevention: the new role of cellular retinol binding proteins. Biomed Res Int. 2015; 2015:624627.
Article19. Fritz H, Flower G, Weeks L, et al. Intravenous vitamin C and cancer: A systematic review. Integr Cancer Ther. 2014; 13:280–300.20. Garland CF, Garland FC, Gorham ED, et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006; 96:252–261.
Article21. Lamson DW, Plaza SM. The anticancer effects of vitamin K. Altern Med Rev. 2003; 8:303–318.22. Frank J. Beyond vitamin E supplementation: an alternative strategy to improve vitamin E status. J Plant Physiol. 2005; 162:834–843.
Article23. Azzi A, Ricciarelli R, Zingg JM. Non-antioxidant molecular functions of alpha-tocopherol (vitamin E). FEBS Lett. 2002; 519:8–10.24. Diplock AT. Safety of antioxidant vitamins and beta-carotene. Am J Clin Nutr. 1995; 62:1510s–1516s.
Article25. Müller L, Theile K, Böhm V. In vitro antioxidant activity of tocopherols and tocotrienols and comparison of vitamin E concentration and lipophilic antioxidant capacity in human plasma. Mol Nutr Food Res. 2010; 54:731–742.
Article26. Prasad KN, Kumar B, Yan XD, et al. Alpha-tocopheryl succinate, the most effective form of vitamin E for adjuvant cancer treatment: a review. J Am Coll Nutr. 2003; 22:108–117.
Article27. Futakuchi M, Fukamachi K, Suzui M. Heterogeneity of tumor cells in the bone microenvironment: mechanisms and therapeutic targets for bone metastasis of prostate or breast cancer. Adv Drug Deliv Rev. 2016; 99:206–211.
Article28. Clezardin P, Teti A. Bone metastasis: pathogenesis and therapeutic implications. Clin Exp Metastasis. 2007; 24:599–608.
Article29. Hauschka PV, Chen TL, Mavrakos AE. Polypeptide growth factors in bone matrix. Ciba Found Symp. 1988; 136:207–225.
Article30. Monteiro AC, Leal AC, Gonçalves-Silva T, et al. T cells induce pre-metastatic osteolytic disease and help bone metastases establishment in a mouse model of metastatic breast cancer. PLoS One. 2013; 8:e68171.31. Tan W, Zhang W, Strasner A, et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011; 470:548–553.
Article32. Chechlinska M, Kowalewska M, Nowak R. Systemic inflammation as a confounding factor in cancer biomarker discovery and validation. Nat Rev Cancer. 2010; 10:2–3.
Article33. Okazaki R, Watanabe R, Inoue D. Osteoporosis associated with chronic obstructive pulmonary disease. J Bone Metab. 2016; 23:111–120.
Article34. Ha H, Lee JH, Kim HN, et al. alpha-Tocotrienol inhibits osteoclastic bone resorption by suppressing RANKL expression and signaling and bone resorbing activity. Biochem Biophys Res Commun. 2011; 406:546–551.
Article35. Simmons JK, Hildreth BE 3rd, Supsavhad W, et al. Animal models of bone metastasis. Vet Pathol. 2015; 52:827–841.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- alpha-Tocopheryl Succinate Inhibits Osteoclast Formation by Suppressing Receptor Activator of Nuclear Factor-kappaB Ligand (RANKL) Expression and Bone Resorption
- Isoliquiritigenin Inhibits Metastatic Breast Cancer Cell-induced Receptor Activator of Nuclear Factor Kappa-B Ligand/Osteoprotegerin Ratio in Human Osteoblastic Cells
- Dissecting Tumor-Stromal Interactions in Breast Cancer Bone Metastasis
- Hypoxia Inducible Factor-1α Directly Induces the Expression of Receptor Activator of Nuclear Factor-κB Ligand in Chondrocytes
- Expression of Osteoprotegerin and RANK Ligand in Breast Cancer Bone Metastasis